��Ŀ����
ú����Ҫ����Դ��Ҳ������������Ʒ����Ҫԭ�ϡ�������ѧ֪ʶ������������⣺
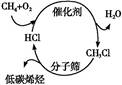
��1��ú��ת����������ú������������Һ��������ú��Һ�������ַ�Ϊ ��
��2����úȼ��ǰ���ú������������ú��ij����������ԭ������ͼ��ʾ��
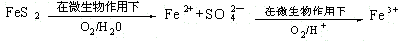
������������Ϊ�������������ü����ĵ�һ����Ӧ�����ӷ���ʽΪ ���ڶ�����Ӧ�����ӷ���ʽΪ ��
��3����ҵú����õ��IJ�Ʒ�н�̿�� �� �ȡ�
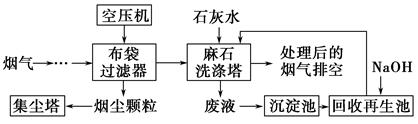
��4��ʪʽʯ��ʯ��ʯ�෨��������������������������һ�ַ������乤�������ǣ���������¯Ԥ�������������������������ַ�ú���̳����پ���һ��ר�ŵ��Ƚ�������Ȼ������������������е�SO2�뺬��ʯ��ʯ�Ľ�Һ������Һ�Ӵ���ͨ�����������ʯ�࣬�������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
��д��ʪ��ʯ��ʯ��ʯ�෨�������漰�Ļ�ѧ��Ӧ����ʽ��______________________��
����ʯ��ʯ��Һ��SO2���ռ���������ʯ������SO2��ԭ���ǣ�_________________________
�����������еõ���ʯ�࣬������Ȼ�����(��Ҫ��Դ��ȼ��ú)�������ʼ���ֵ����ʯ���Ʒ���ܱ仵����ҵ�������������Ȼ���ķ�����____________________________________ ��
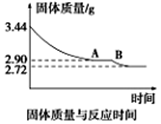
��5��ij��ѧ��ȤС��Ϊ�˲ⶨ������������ʯ������(CaSO4��xH2O)���ⶨxֵ��������ʵ�飺��ʯ�����ʹ֮��ˮ�����ȹ����й����������ʱ��ı仯��ϵ��ͼ��ʾ�����ݱ��������������Ϊ2��72g���ٸı䡣��ʯ��Ļ�ѧʽΪ_______________����ͼ����AB�ζ�Ӧ������Ļ�ѧʽΪ_________________��
��1��ú��ת����������ú������������Һ��������ú��Һ�������ַ�Ϊ ��
��2����úȼ��ǰ���ú������������ú��ij����������ԭ������ͼ��ʾ��
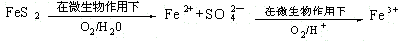
������������Ϊ�������������ü����ĵ�һ����Ӧ�����ӷ���ʽΪ ���ڶ�����Ӧ�����ӷ���ʽΪ ��
��3����ҵú����õ��IJ�Ʒ�н�̿�� �� �ȡ�
��4��ʪʽʯ��ʯ��ʯ�෨��������������������������һ�ַ������乤�������ǣ���������¯Ԥ�������������������������ַ�ú���̳����پ���һ��ר�ŵ��Ƚ�������Ȼ������������������е�SO2�뺬��ʯ��ʯ�Ľ�Һ������Һ�Ӵ���ͨ�����������ʯ�࣬�������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
��д��ʪ��ʯ��ʯ��ʯ�෨�������漰�Ļ�ѧ��Ӧ����ʽ��______________________��
����ʯ��ʯ��Һ��SO2���ռ���������ʯ������SO2��ԭ���ǣ�_________________________
�����������еõ���ʯ�࣬������Ȼ�����(��Ҫ��Դ��ȼ��ú)�������ʼ���ֵ����ʯ���Ʒ���ܱ仵����ҵ�������������Ȼ���ķ�����____________________________________ ��
��5��ij��ѧ��ȤС��Ϊ�˲ⶨ������������ʯ������(CaSO4��xH2O)���ⶨxֵ��������ʵ�飺��ʯ�����ʹ֮��ˮ�����ȹ����й����������ʱ��ı仯��ϵ��ͼ��ʾ�����ݱ��������������Ϊ2��72g���ٸı䡣��ʯ��Ļ�ѧʽΪ_______________����ͼ����AB�ζ�Ӧ������Ļ�ѧʽΪ_________________��

��1��ֱ��Һ������ ���Һ������
��2��2FeS2+7O2+2H2O=4H++2Fe2++4SO42- 4Fe2++O2+4H+=4Fe3++2H2O
��3����¯ú�����ְ�ˮ��ú���ͣ�
��4����CaCO3+SO2=CaSO3+CO2 2CaSO3+O2=2CaSO4
����ʯ��ʯ��Һ�ijɱ��ϵ� ����ˮϴ��
��5����CaSO4��2H2O ��2CaSO4�� H2O
��2��2FeS2+7O2+2H2O=4H++2Fe2++4SO42- 4Fe2++O2+4H+=4Fe3++2H2O
��3����¯ú�����ְ�ˮ��ú���ͣ�
��4����CaCO3+SO2=CaSO3+CO2 2CaSO3+O2=2CaSO4
����ʯ��ʯ��Һ�ijɱ��ϵ� ����ˮϴ��
��5����CaSO4��2H2O ��2CaSO4�� H2O
�����������1��ú��Һ�������ַ�Ϊֱ��Һ�������ͼ��Һ�������������͡���2���ɿ�ͼת����ϵ��������غ㶨�ɿ�֪���������������ĵ�һ����Ӧ�����ӷ���ʽΪ2FeS2+7O2+2H2O= 4H++2Fe2++ 4SO42-���ڶ�����Ӧ�����ӷ���ʽΪ4Fe2++O2+4H+=4Fe3++2H2O����3���ڹ�ҵ��ú����õ��IJ�Ʒ�н�̿��ú���͡���¯ú�����ְ�ˮ�ȡ���4����ʪ��ʯ��ʯ��ʯ�෨�������漰�Ļ�ѧ��Ӧ����ʽΪ��CaCO3+ SO2
 CaSO3+CO2 2CaSO3+O2
CaSO3+CO2 2CaSO3+O2 2CaSO4������ʯ��ʯ��Һ��SO2���ռ���������ʯ������SO2����Ϊ��ʯ��ʯ��Һ�ijɱ��ϵͣ�Ч��ߡ������������еõ���ʯ�࣬������Ȼ�����(��Ҫ��Դ��ȼ��ú)�������ʼ���ֵ����ʯ���Ʒ���ܱ仵������CaSO4����ˮ����CaCl2�����ܽ���ˮ�����Թ�ҵ�������������Ȼ���ķ�������ˮϴ�ӡ���5����n(CaSO4)=2��72g��136g/mol=0��02mol; n(H2O)=(3��44g-2��72g)��18g/mol=0��04mol�� n(CaSO4): n(H2O)=1:2������ʯ��Ļ�ѧʽΪCaSO4��2H2O����n(H2O)=(2��90g-2��72g) ��18g/mol=0��01mol, n(CaSO4): n(H2O)= 0��02mol:0��01mol=2:1,����ͼ����AB�ζ�Ӧ������Ļ�ѧʽΪ2CaSO4�� H2O��
2CaSO4������ʯ��ʯ��Һ��SO2���ռ���������ʯ������SO2����Ϊ��ʯ��ʯ��Һ�ijɱ��ϵͣ�Ч��ߡ������������еõ���ʯ�࣬������Ȼ�����(��Ҫ��Դ��ȼ��ú)�������ʼ���ֵ����ʯ���Ʒ���ܱ仵������CaSO4����ˮ����CaCl2�����ܽ���ˮ�����Թ�ҵ�������������Ȼ���ķ�������ˮϴ�ӡ���5����n(CaSO4)=2��72g��136g/mol=0��02mol; n(H2O)=(3��44g-2��72g)��18g/mol=0��04mol�� n(CaSO4): n(H2O)=1:2������ʯ��Ļ�ѧʽΪCaSO4��2H2O����n(H2O)=(2��90g-2��72g) ��18g/mol=0��01mol, n(CaSO4): n(H2O)= 0��02mol:0��01mol=2:1,����ͼ����AB�ζ�Ӧ������Ļ�ѧʽΪ2CaSO4�� H2O��
��ϰ��ϵ�д�
 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
�����Ŀ