��Ŀ����
����Ŀ������Cu2O�������Ĵ����Ͱ뵼����ϣ���ҵ�ϳ������з����Ʊ�Cu2O��
��1���Ȼ�ԭ��
���������£���Һ̬�£�N2H4����ԭ���Ƶ�Cu(OH��2�Ʊ�Cu2O��ͬʱ�ų�N2���÷�Ӧ�Ļ�ѧ����ʽΪ________________��
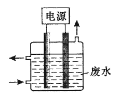
��2����ⷨ ������ȼ�ϵ��Ϊ��Դ���õ�ⷨ�Ʊ�Cu2O��װ����ͼ��

��A�Ļ�ѧʽΪ________________��
��ȼ�ϵ���У�OH-���ƶ�����Ϊ________________���������������������������������������У������ĵ缫��ӦʽΪ________________��
�����һ��ʱ�����ʹ��������Һ�ָ�ԭ����ɣ�Ӧ�����в���һ����________________(�ѧʽ����
���Ʊ������У���ѭ�����õ�����Ϊ________________���ѧʽ����
��3���ɷ���ԭ��
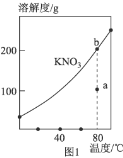
���÷�ӦCu +CuO![]() Cu2O�Ʊ�Cu2O������Ӧ��ľ��ȹ�������������ֳɷ֣��ȷ�Ϊ���ݣ�һ��������H2��ַ�Ӧ������������6.4g����һ��ǡ������500mLϡ���ᣬ���ɱ�״����4.48LNO����ϡ��������ʵ���Ũ��Ϊ________��
Cu2O�Ʊ�Cu2O������Ӧ��ľ��ȹ�������������ֳɷ֣��ȷ�Ϊ���ݣ�һ��������H2��ַ�Ӧ������������6.4g����һ��ǡ������500mLϡ���ᣬ���ɱ�״����4.48LNO����ϡ��������ʵ���Ũ��Ϊ________��
���𰸡�4Cu (OH��2+N2H4![]() N2+2Cu2O+6H2O O2 �������� 2Cu+2OH--2e-=Cu2O+H2O H2O H2 3.2mol/L
N2+2Cu2O+6H2O O2 �������� 2Cu+2OH--2e-=Cu2O+H2O H2O H2 3.2mol/L
��������
��1���ڼ��������£���Һ̬�£�N2H4����ԭ���Ƶ�Cu(OH��2�Ʊ�Cu2O��ͬʱ�ų�N2������ԭ���غ㡢�����غ�ɵø÷�Ӧ�Ļ�ѧ����ʽΪ4Cu(OH��2+N2H4![]() N2+2Cu2O+6H2O����2����������ȼ�ϵ��Ϊ��Դ���õ�ⷨ�Ʊ�Cu2O����Cu�缫���Դ���������ӣ���ȼ�ϵ��ͨ�����ļ�Ϊ������ͨ�������ĵ缫Ϊ���������Կ�֪����AΪ������A�Ļ�ѧʽΪO2 ��
N2+2Cu2O+6H2O����2����������ȼ�ϵ��Ϊ��Դ���õ�ⷨ�Ʊ�Cu2O����Cu�缫���Դ���������ӣ���ȼ�ϵ��ͨ�����ļ�Ϊ������ͨ�������ĵ缫Ϊ���������Կ�֪����AΪ������A�Ļ�ѧʽΪO2 ��
������ͬ�ֵ����ų⣬���ֵ���������ԭ����ԭ��������������ƶ�����ȼ�ϵ����OH-���ƶ�����Ϊ���������ƶ����ڵ�����������ͭ����������Cu+��Ȼ������Һ�е�OH-����γ�Cu2O���缫��ӦʽΪ2Cu+ 2OH-- 2e-=Cu2O+H2O��
�������ڷ������ܷ�ӦʽΪ2Cu+H2O![]() Cu2O+H2������֪����Һ�м�����ˮ����ɣ�����Ҫ�����������ˮ����ѧʽ��H2O��
Cu2O+H2������֪����Һ�м�����ˮ����ɣ�����Ҫ�����������ˮ����ѧʽ��H2O��
�����������õ��������ɲ���ȼ�ϵ���У���Ϊȼ�����ĵ��������ɼ���ѭ��������ΪH2�� ��3�� ��Cu2O���ΪCu��CuO��ԭ������Cu��CuO�Ļ����,��һ�ݻ������������������ԭ���õ�Cu��ˮ����Ӧ�������������6.40gΪ�������OԪ�ص�������Oԭ�ӵ����ʵ���Ϊn(O)=6.4g��16g/mol=0.4mol������CuԪ���غ��֪n(CuO��= n(O)=0.4mol����һ���м���500mLϡ���ᣬ����ǡ����ȫ�ܽ⣬��Һ������ΪCu(NO3)2����ͬʱ�ռ�����״����NO����4.48L��NO�����ʵ���Ϊn(NO��=4.48L��22.4L/mol=0.2mol�����ݵ���ת���غ��֪�ڲ�ֺ�Cu��CuO�Ļ������,2n(Cu��=3n(NO��=3��0.2mol��n (Cu��=0.3mol����ͭԪ���غ��֪n[Cu(NO3)2]=n��CuO��+n��Cu��=0.4mol+0.3mol=0.7mol�����ݵ�Ԫ���غ��֪n(HNO3)= n(NO)+2 n[Cu(NO3)2] =0.2mol+2��0.7mol=1.6mol�������Ũ��Ϊ1.6mol��0.5L=3.2 mol/L��