��Ŀ����
����Ŀ��ij����С���ͬѧ��ʵ������п��Ũ���ᷴӦ��ʵ���У���ͬѧ��Ϊ�����������Ƕ���������ͬѧ��Ϊ���������������⣬�����ܲ���������Ϊ����֤��λͬѧ���ж���ȷ����ͬѧ�����ͼ�е�ʵ��װ��(п��Ũ���Ṳ��ʱ����������ΪX���Ҹ÷�Ӧװ����ȥ)��
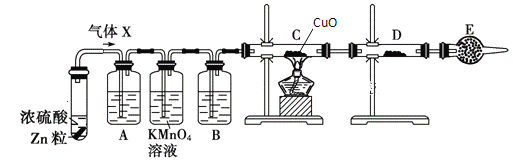
�Իش��������⣺
��1��������Ӧ�����ɶ�������Ļ�ѧ����ʽΪ_______
��2��A�м�����Լ�������_____��������_____��B�м�����Լ�������_____��������_____��E�м�����Լ�������_____��������_____
��3������֤������X�к���������ʵ������C��______��D��____��
���𰸡�Zn+2H2SO4(Ũ)![]() ZnSO4+SO2��+2H2O Ʒ����Һ ����SO2 Ũ���� ����ˮ���� ��ʯ�� ��ֹ�����е�ˮ��������װ��D�� ��ɫ��ĩ��ɺ�ɫ ��ɫ��ĩ�����ɫ
ZnSO4+SO2��+2H2O Ʒ����Һ ����SO2 Ũ���� ����ˮ���� ��ʯ�� ��ֹ�����е�ˮ��������װ��D�� ��ɫ��ĩ��ɺ�ɫ ��ɫ��ĩ�����ɫ
��������
��1��Ũ������ǿ�����ԣ���п��ԭ�����ɶ�������Ӧ��ѧ����ʽΪ��Zn+2H2SO4(Ũ)![]() ZnSO4+SO2��+2H2O��
ZnSO4+SO2��+2H2O��
��2������ʵ��ԭ����װ�÷����ã�ʵ��������Ʒ����Һ�������ɵĶ�������Ȼ�������Ը��������Һ��ȥ����������Ũ��������������ͭ��������������ˮ����ͭ�������ɵ�ˮ��Ϊ�˷�ֹ������ˮ�ֵĸ��ţ���װ��ĩ��ʹ�ø���ܸ������A�м�����Լ�������Ʒ����Һ�������Ǽ���SO2��B�м�����Լ�������Ũ���ᣬ����������ˮ������E�м�����Լ������Ǽ�ʯ�ң������Ƿ�ֹ�����е�ˮ��������װ��D��
��3����������ԭ����ͭʵ����������Ĵ��ڣ�����C������Ϊ��ɫ��ĩ��ɺ�ɫ����Ӧ����ˮ��ʹD�еİ�ɫ��ˮ����ͭ��ĩ�����ɫ��


