��Ŀ����
��12�֣��±������ڱ��е�һ���֣�����A��I�����ڱ��е�λ�ã���Ԫ�ط��Ż�ѧʽ�ش���������:
��1������ЩԪ���У�����õĽ���Ԫ���� ������õ�Ԫ���� ��
��2������ЩԪ�ص�����������Ӧˮ�����У�������ǿ���� �������Ե����������� ��
��3��д��CԪ�صĵ�����B����������ˮ���ﷴӦ�����ӷ���ʽ ��
��4��E���⻯��Ļ�ѧʽΪ
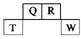
| �� ���� | IV | IIV | IIIV | IVA | VA | VIA | VIIA | O |
| 1 | A | | | | | | | |
| 2 | | | | D | E | | G | I |
| 3 | B | | C | | F | | H | |
��2������ЩԪ�ص�����������Ӧˮ�����У�������ǿ���� �������Ե����������� ��
��3��д��CԪ�صĵ�����B����������ˮ���ﷴӦ�����ӷ���ʽ ��
��4��E���⻯��Ļ�ѧʽΪ
��1��Na Ne ��2��HClO4 Al (OH)3
��3��2Al+2OH��+2H2O=2AlO2��+3H2�� ��4��NH3
��3��2Al+2OH��+2H2O=2AlO2��+3H2�� ��4��NH3
����Ԫ�����ڱ��Ľṹ��Ԫ�������ɵ�Ӧ�á�����Ԫ�������ڱ��е�λ�ÿ��жϣ�A��H��B��Na��C��Al��D��C��E��N��F��P��G��F��H��Cl��I��Ne��ͬ����Ԫ���������ҽ������������ǽ���������ǿ��ͬ����Ԫ�����϶��½���������ǿ���ǽ�����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ