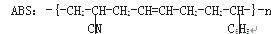��Ŀ����
��18�֣�B��һ����Ԫ��״�������˴Ź�������ֻ��һ���壻H�ĺ˴Ź���������3���壬�����֮��Ϊ2��2��1��I��һ�ֺϳ�����֬����Ҫԭ�ϡ���֪R���������й�����ת����ϵ����Ϣ���£�
�밴Ҫ��ش��������⣺
��1��A�IJ�����������Ϊ _______________���۷�Ӧ����Ϊ____________________��
��2��B�Ľṹ��ʽΪ __________________��H��ϵͳ������Ӧ����Ϊ________________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��____________________________________________________________________��
�� ____________________________________________________________________��
��4��д��˳���Ľṹ��ʽ_____________________________��˳�����Ըߣ��ͺ��Ժã����л������������͵��£��ϳ�·�����£�
���ȹ���(CH3)2SiCl2 ��������� (CH3)2Si(OH)2
��������� (CH3)2Si(OH)2 ����
����
��д�����۵Ļ�ѧ����ʽ__________________________________________________________��
��5���л���J��F��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ������������Ŀ��д�����з���������J�Ľṹ��ʽ��������F����_________________________________________________________________��
��1�� -Br(��ԭ��)��1�֣� ��ԭ��Ӧ��1�֣���2��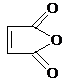 ��2�֣�1��4-��������2�֣�
��2�֣�1��4-��������2�֣�
��3���� 2HOOCCH2CHOHCOOH 2H2O+
2H2O+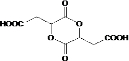 ��2�֣�
��2�֣�
��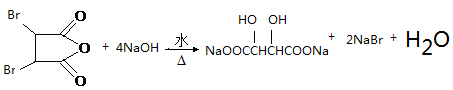 (2�֣�
(2�֣�
��4��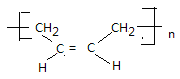 ��2�֣�
��2�֣�
n(CH3)2Si(OH)2��(n-1)H2O + ��2�֣�
��2�֣�
��5�� ��2�� 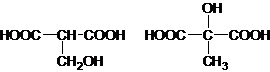
�������������B��һ����Ԫ��״�������˴Ź�������ֻ��һ���壬�������巢���ӳɷ�Ӧ��˵�������к���̼̼˫����ӦΪ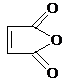 ��Bˮ������C��C�Ľṹ��ʽΪHOOCCH��CHCOOH��C��ˮ������Ӧ����F������C��F��Ħ��������֪��C��ˮ�����ӳɷ�Ӧ����F������F�Ľṹ��ʽΪ��HOOCCH2CH��OH��COOH��C�����������ӳɷ�Ӧ����D��D�Ľṹ��ʽΪHOOCCH2CH2COOH����������Ϣ֪��H�Ľṹ��ʽΪHOCH2CH2CH2CH2OH��IΪCH2��CHCH��CH2��GΪ
��Bˮ������C��C�Ľṹ��ʽΪHOOCCH��CHCOOH��C��ˮ������Ӧ����F������C��F��Ħ��������֪��C��ˮ�����ӳɷ�Ӧ����F������F�Ľṹ��ʽΪ��HOOCCH2CH��OH��COOH��C�����������ӳɷ�Ӧ����D��D�Ľṹ��ʽΪHOOCCH2CH2COOH����������Ϣ֪��H�Ľṹ��ʽΪHOCH2CH2CH2CH2OH��IΪCH2��CHCH��CH2��GΪ ��AΪ
��AΪ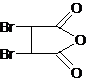 ��EΪNaOOCCHOHCHOHCOONa����
��EΪNaOOCCHOHCHOHCOONa����
��1��AΪ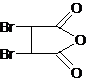 ������������Ϊ-Br����Ӧ�����Ȼ����ǻ�������ȥ���ķ�Ӧ������ǻ�ԭ��Ӧ��
������������Ϊ-Br����Ӧ�����Ȼ����ǻ�������ȥ���ķ�Ӧ������ǻ�ԭ��Ӧ��
��2�������Ϸ�����֪BΪ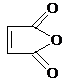 ��H�Ľṹ��ʽΪHOCH2CH2CH2CH2OH��Ϊ1��4-��������
��H�Ľṹ��ʽΪHOCH2CH2CH2CH2OH��Ϊ1��4-��������
��3����Ӧ�ߵĻ�ѧ����ʽΪ2HOOCCH2CHOHCOOH 2H2O+
2H2O+ ����Ӧ����±������ˮ�ⷴӦ����Ӧ�Ļ�ѧ����ʽΪ
����Ӧ����±������ˮ�ⷴӦ����Ӧ�Ļ�ѧ����ʽΪ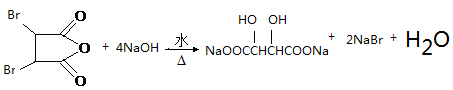 ��
��
��4��˳��1,3������ϩ�����Ӿ۷�Ӧ������˳��ϩ���ṹ��ʽΪ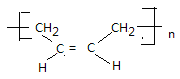 ���������������2���ǻ����������۷�Ӧ���ɹ�����Ӧ�Ļ�ѧ����ʽΪn(CH3)2Si(OH)2��(n-1)H2O +
���������������2���ǻ����������۷�Ӧ���ɹ�����Ӧ�Ļ�ѧ����ʽΪn(CH3)2Si(OH)2��(n-1)H2O + ��
��
��5��F�Ľṹ��ʽΪ��HOOCCH2CH��OH��COOH����Ӧ�ĺ�����ͬ�����ŵ�ͬ���칹����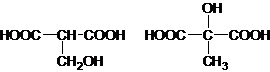 ��
��
���㣺�����л����ƶϡ������š��л���Ӧ���͡�������ͬ���칹���жϡ��Լ�����ʽ��д�ĵ�

����15�֣�ҩ��Z����������������ϵͳ�Ժ���Ǵ��ȣ�����X��1,4-������ͪ���Ҷ�����ͪ����Y�������ᣩΪԭ�Ϻϳɣ�����ͼ��
��1��������X���� �ֻ�ѧ������ͬ����ԭ�ӡ�
��2������˵����ȷ���� ��
| A��X�Ƿ��㻯���� | B��Ni����Y����5molH2�ӳ� |
| C��Z�ܷ����ӳɡ�ȡ������ȥ��Ӧ | D��1mol Z������5mol NaOH��Ӧ |
��4��X������ ��д���ƣ���M��
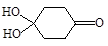 �����Ӽ���ˮ���ã�һ�������£�M����1����OH����ȥ��Ӧ�õ��ȶ�������N������ʽΪC6H8O2������N�Ľṹ��ʽΪ ����֪ϩ��ʽ���ȶ����ᷢ���������ţ����磺
�����Ӽ���ˮ���ã�һ�������£�M����1����OH����ȥ��Ӧ�õ��ȶ�������N������ʽΪC6H8O2������N�Ľṹ��ʽΪ ����֪ϩ��ʽ���ȶ����ᷢ���������ţ����磺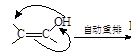
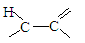 ����
������5��YҲ�����뻷�����飨
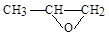 ���������Ʒ�Ӧ�ٵķ�Ӧ����������Ľṹ��ʽΪ ��дһ�֣���Y��ͬ���칹��ܶ��֣������б�����������������ȡ�������ҷ��ǻ���λ�ú���Ŀ�����䣩����������ͬ���칹���� �֡�
���������Ʒ�Ӧ�ٵķ�Ӧ����������Ľṹ��ʽΪ ��дһ�֣���Y��ͬ���칹��ܶ��֣������б�����������������ȡ�������ҷ��ǻ���λ�ú���Ŀ�����䣩����������ͬ���칹���� �֡� ij�о�С���Լױ�Ϊ��Ҫԭ�ϣ���������·�ߺϳ�ҽҩ�м���F��Y��
��֪����)  ������
������ ��
��
��ش��������⣺
��1��д��Y�к��������ŵ����� ��
��2�������й�F��˵����ȷ���� ��
| A������ʽ��C7H7NO2Br | B�����������ᷴӦ������NaOH��Һ��Ӧ |
| C���ܷ���������Ӧ | D��1 mol F����������2 mol NaOH |
��4���ںϳ�F�Ĺ����У�B��C���費��ʡ�ԣ������� ��
F��һ���������γɵĸ߷��ӻ�����Ľṹ��ʽ�� ��
��5��д��һ��ͬʱ��������������Y��ͬ���칹��Ľṹ��ʽ ��
�ٱ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�� ��������Ũ��ˮ��Ӧ������ɫ����
�۷�����ֻ����һ����״�ṹ
��6����X����ϩΪԭ�Ͽɺϳ�Y����д������Ƶĺϳ�·�ߣ����Լ����ܼ���ѡ����
�ϳ�·�ߵ���д��ʽ���£�
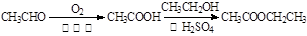



 �ϳ�
�ϳ� (�����Լ��ͷ�Ӧ��������ȥ)��
(�����Լ��ͷ�Ӧ��������ȥ)��
 ������______������ͬ�����ԡ��������ԡ������ԡ���
������______������ͬ�����ԡ��������ԡ������ԡ���
 ת��Ϊ
ת��Ϊ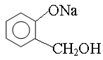 ����Ӧ����____________��
����Ӧ����____________��