��Ŀ����
��2011?��ɽ��ģ�⣩���������ϣ���������AlN���մ���һ������ʯ��������������ǽ������ϣ���߿��ȶ���2200�森�����Ժã�������ϵ��С�������õ����ȳ�����ϣ������ڽ�����ʴ������ǿ���������������������Ͻ�������������ϣ����������ǵ��Ե�壬����������ã���������Ԫ��Ҳ����ϣ������ϸ��������ĩ���㷺Ӧ���ڴ��ģ���ɵ�·������������ȡԭ��Ϊ��
Al2O3+3C+N2
2AlN+3CO
������̽����ij��ѧ�о���ѧϰС���Ա���ݵ���������ȡԭ��������������̽����
����1������ȡ������ʱ���ڷ�Ӧ����ȫ����������Ʒ���������ʳ���̼������ܴ���
����2��Ϊ�ⶨ�ò�Ʒ���йسɷֵĺ������ס�����ͬѧ�������������ʵ�飺
��1����ͬѧ����ȡ10.00g��Ʒ������������������������Һ�й��Ȳ����ɣ�AlN������������Һ��Ӧ����NaAlO2�����ų�����3.36L����״������
��������Ӧ�Ļ�ѧ����ʽΪ
�ڸ���Ʒ�е�AlN����������Ϊ
��2����ͬѧ����ȡ10.00g��Ʒ���ڷ�Ӧ���У�ͨ��2.016L����״����O2���ڸ����³�ַ�Ӧ����������ܶ�Ϊ1.34g?L-1�����۳ɱ�״����AlN����O2��Ӧ��������Ʒ�к�����̼
����3����ͬѧ�ܵ��ס���ͬѧʵ�����������Ϊ�ⶨij�������к���̼�����������ʣ�����ͼ��I��һЩװ�������м��飬����AlN��NaOH��Һ��Ӧ�����ɰ�����������ⶨ��Ʒ�е�����������������������ʵ��������ȷ�����ʵijɷ֣�ʵ���е���������Բ��ƣ�
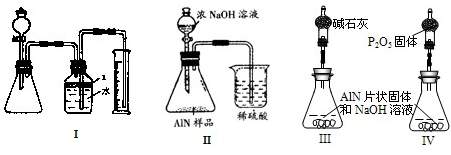
��1��ʵ���йز���Ϊ��������ƿ�з���������AlN��Ʒ���ڴӷ�Һ©������ƿ�м��������ŨNaOH���ۼ���װ�õ������ԣ��ܲⶨ�ռ���ˮ�������
��ȷ�IJ���˳��Ϊ
��2���������м��װ�������Եķ�����
��3�����ƿ�е��Լ�X�����ѡ��
A���� B���ƾ� C��ֲ���� D��CCl4
��4�����ƿ��Һ��û��װ�����Ϸ����������ռ䣩��ʵ����NH3�������
��5����ʵ���в����Ʒ������Ϊwg�����������ΪaL������£�������Ʒ��AlN����������Ϊ
%
%��AlN��ʽ��Ϊ41����
��6��ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�к��е�������
����4����ͬѧ��Ϊ����ͬѧ��ʵ�鷽��������������������������������ϴ������ͼ9�еĢ�װ�ý���ͬ��ʵ�飬ͨ���ⶨ�ձ��������������ȷ����Ʒ��AlN����������������Ϊ�Ƿ���У�
����5����ͬѧ��ϸ˼���˶�ͬѧ��װ�ú���Ϊ��װ�������õ���Ʒ��AlN����ƫС����ԭ����
Al2O3+3C+N2
| ���� |
������̽����ij��ѧ�о���ѧϰС���Ա���ݵ���������ȡԭ��������������̽����
����1������ȡ������ʱ���ڷ�Ӧ����ȫ����������Ʒ���������ʳ���̼������ܴ���
������
������
������2��Ϊ�ⶨ�ò�Ʒ���йسɷֵĺ������ס�����ͬѧ�������������ʵ�飺
��1����ͬѧ����ȡ10.00g��Ʒ������������������������Һ�й��Ȳ����ɣ�AlN������������Һ��Ӧ����NaAlO2�����ų�����3.36L����״������
��������Ӧ�Ļ�ѧ����ʽΪ
AlN+NaOH+H2O=NaAlO2+NH3��
AlN+NaOH+H2O=NaAlO2+NH3��
���ڸ���Ʒ�е�AlN����������Ϊ
61.5%
61.5%
����2����ͬѧ����ȡ10.00g��Ʒ���ڷ�Ӧ���У�ͨ��2.016L����״����O2���ڸ����³�ַ�Ӧ����������ܶ�Ϊ1.34g?L-1�����۳ɱ�״����AlN����O2��Ӧ��������Ʒ�к�����̼
1.92
1.92
g������3����ͬѧ�ܵ��ס���ͬѧʵ�����������Ϊ�ⶨij�������к���̼�����������ʣ�����ͼ��I��һЩװ�������м��飬����AlN��NaOH��Һ��Ӧ�����ɰ�����������ⶨ��Ʒ�е�����������������������ʵ��������ȷ�����ʵijɷ֣�ʵ���е���������Բ��ƣ�

��1��ʵ���йز���Ϊ��������ƿ�з���������AlN��Ʒ���ڴӷ�Һ©������ƿ�м��������ŨNaOH���ۼ���װ�õ������ԣ��ܲⶨ�ռ���ˮ�������
��ȷ�IJ���˳��Ϊ
�ۢ٢ڢ�
�ۢ٢ڢ�
����2���������м��װ�������Եķ�����
�رշ�Һ©������������ƿ�����ƿ���Ҳർ��ˮ������������ʱˮ����������
�رշ�Һ©������������ƿ�����ƿ���Ҳർ��ˮ������������ʱˮ����������
����3�����ƿ�е��Լ�X�����ѡ��
C
C
����ѡ��ı�ţ���A���� B���ƾ� C��ֲ���� D��CCl4
��4�����ƿ��Һ��û��װ�����Ϸ����������ռ䣩��ʵ����NH3�������
����
����
����ƫ��ƫС�䣩����5����ʵ���в����Ʒ������Ϊwg�����������ΪaL������£�������Ʒ��AlN����������Ϊ
| 4100a |
| 22.4w |
| 4100a |
| 22.4w |
��6��ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�к��е�������
̼
̼
��Ϊ�˲ⶨ�Ƿ����������ʣ�����Ҫ��Щ������̼������
̼������
������4����ͬѧ��Ϊ����ͬѧ��ʵ�鷽��������������������������������ϴ������ͼ9�еĢ�װ�ý���ͬ��ʵ�飬ͨ���ⶨ�ձ��������������ȷ����Ʒ��AlN����������������Ϊ�Ƿ���У�
������
������
�����롰���С����������С�����ԭ����II��NH3���ױ����գ�������������ͬʱ�����к���ˮ������Ӱ�찱�������IJⶨ
II��NH3���ױ����գ�������������ͬʱ�����к���ˮ������Ӱ�찱�������IJⶨ
����ĸĽ�����Ϊ��װ��֮������ʢ�м�ʯ�ҵĸ���ܣ��ձ����ܵ�ĩ�˽�һ���۵�©�������հ���
��װ��֮������ʢ�м�ʯ�ҵĸ���ܣ��ձ����ܵ�ĩ�˽�һ���۵�©�������հ���
������5����ͬѧ��ϸ˼���˶�ͬѧ��װ�ú���Ϊ��װ�������õ���Ʒ��AlN����ƫС����ԭ����
��Ӧ�����İ��������ܱ���ȫ����
��Ӧ�����İ��������ܱ���ȫ����
�������Դ�ԭ��Ļ���ֻҪ��ͼ9�е�III��IV����װ���е�һ�֣�ֻ����м��ֱ�Ҫ�����ݲⶨ���ɱȽ�ȷ��ȷ����Ʒ��AlN�������������Ϻ�����װ��Ϊ��
��
������ţ�������Ϊ��ͬѧ��װ���Ƿ���ȱ�ݣ���
��
�����У���������ƫ��ƫ��ƫ��
ƫ��
��Ӧ����θĽ���Ӧ�ٽ�һ����IVװ������ȫ��ͬ�ĸ���ܣ����ٽ�һ��װ�м�ʯ�ҵĸ����Ҳ�У�
Ӧ�ٽ�һ����IVװ������ȫ��ͬ�ĸ���ܣ����ٽ�һ��װ�м�ʯ�ҵĸ����Ҳ�У�
��������ȱ�ݺ����˸�ɲ��������������1���������ʲ�һ����ȫ��Ӧ�����жϣ�
����2����1��������Ŀ��Ϣ��֪������AlN������������Һ��Ӧ����NaAlO2�����ų�NH3���ݴ����ɷ���ʽ��
������0.15molNH3�����ݵ�Ԫ���غ��֪10.00g��Ʒ�к���AlN�����ʵ���Ϊ0.15mol������m=nM����AlN���������ٸ�����������������㣮
��2����Ӧ�������CO2��CO��CO2��CO��CO2��O2�Ļ�����壬�������������ܶȿɼ������������ƽ����Է�������Ϊ30��������������ض���CO2��CO������壮���������к���CO2xmol��CO ymol��������ԭ���غ��ƽ����Է��������ļ��㣬�зų�����x��y��ֵ����Ʒ��n��C��=��x+y��mol���ݴ˼�����Ʒ��̼��������
����3����1�����װ�õ��������������ڼ�ҩƷǰ���У����������ҩƷ���˷ѣ�
��2����������ԵĹؼ���������ϵҪ�����ܱ�״̬�����õķ����У��ܷ������������������������Һ�������γ�Һ��߶Ȳ�������Ϊ���رշ�Һ©������������ƿ�����ƿ���Ҳർ��ˮ������������ʱˮ���������䣮
��3��X�����ϲ㣬�ܶȱ�ˮС���Ҳ�����ˮ�������հ��������ڸ��백����ˮ��ע�����ڱ��ӷ������˷��Լ���Ӱ�찱������ⶨ��
��4�����ƿ�ڵ�Һ���Ƿ������Ӱ�����ռ�����NH3�����С��
��5����������������ΪaL�����ʵ������ٸ��ݷ���ʽ�����AlN�����ʵ�������������AlN���������������������Ķ��������Ʒ��AIN������������
��6������AlN�е����ʿ�����̼��Al2O3����ֻ̼������NaOH��Һ��Ӧ����ʱ�Ĺ�����Ϊ̼��ֻҪ֪��̼�������백������������ݰ����������AlN����������̼��AlN������֮������Ʒ������ϵ������ȷ���Ƿ����������ʣ�
����4��II��NH3���ױ����գ�������������ͬʱ�����к���ˮ������Ӱ�찱�������IJⶨ�����Բ����У������ĸ���Ϊ����װ��֮������ʢ�м�ʯ�ҵĸ���ܣ��ձ����ܵ�ĩ�˽�һ���۵�©�������հ�����
����5��ͨ���ⶨ��������������ʵ���������������������㵪��������������Ϊ��һ���ְ���������ƿ�У���Ӧ�����İ�����ȫ����ϡ�������գ����ʵ����AlN������������ƫС��
��ͬѧ��Ƶ�װ�â�Ϻ���������ͨ����Ӧǰ���������������ⶨAlN����������������ĸ�������������ձ�NH3��������ˮ��������Ӧ���ȣ�������ʹ������ˮ������NH3���ߣ�������IV����NH3�����գ���Ӧǰ���������
����ͬѧ��δ���ǵ������е�ˮ������CO2�Ľ��룬������ɷ�Ӧ��������������൱��NH3�ļ��٣����½��ƫ�ͣ��ʶ�Ӧ�ټ�һ��ͬ���ĸ���ܣ�
����2����1��������Ŀ��Ϣ��֪������AlN������������Һ��Ӧ����NaAlO2�����ų�NH3���ݴ����ɷ���ʽ��
������0.15molNH3�����ݵ�Ԫ���غ��֪10.00g��Ʒ�к���AlN�����ʵ���Ϊ0.15mol������m=nM����AlN���������ٸ�����������������㣮
��2����Ӧ�������CO2��CO��CO2��CO��CO2��O2�Ļ�����壬�������������ܶȿɼ������������ƽ����Է�������Ϊ30��������������ض���CO2��CO������壮���������к���CO2xmol��CO ymol��������ԭ���غ��ƽ����Է��������ļ��㣬�зų�����x��y��ֵ����Ʒ��n��C��=��x+y��mol���ݴ˼�����Ʒ��̼��������
����3����1�����װ�õ��������������ڼ�ҩƷǰ���У����������ҩƷ���˷ѣ�
��2����������ԵĹؼ���������ϵҪ�����ܱ�״̬�����õķ����У��ܷ������������������������Һ�������γ�Һ��߶Ȳ�������Ϊ���رշ�Һ©������������ƿ�����ƿ���Ҳർ��ˮ������������ʱˮ���������䣮
��3��X�����ϲ㣬�ܶȱ�ˮС���Ҳ�����ˮ�������հ��������ڸ��백����ˮ��ע�����ڱ��ӷ������˷��Լ���Ӱ�찱������ⶨ��
��4�����ƿ�ڵ�Һ���Ƿ������Ӱ�����ռ�����NH3�����С��
��5����������������ΪaL�����ʵ������ٸ��ݷ���ʽ�����AlN�����ʵ�������������AlN���������������������Ķ��������Ʒ��AIN������������
��6������AlN�е����ʿ�����̼��Al2O3����ֻ̼������NaOH��Һ��Ӧ����ʱ�Ĺ�����Ϊ̼��ֻҪ֪��̼�������백������������ݰ����������AlN����������̼��AlN������֮������Ʒ������ϵ������ȷ���Ƿ����������ʣ�
����4��II��NH3���ױ����գ�������������ͬʱ�����к���ˮ������Ӱ�찱�������IJⶨ�����Բ����У������ĸ���Ϊ����װ��֮������ʢ�м�ʯ�ҵĸ���ܣ��ձ����ܵ�ĩ�˽�һ���۵�©�������հ�����
����5��ͨ���ⶨ��������������ʵ���������������������㵪��������������Ϊ��һ���ְ���������ƿ�У���Ӧ�����İ�����ȫ����ϡ�������գ����ʵ����AlN������������ƫС��
��ͬѧ��Ƶ�װ�â�Ϻ���������ͨ����Ӧǰ���������������ⶨAlN����������������ĸ�������������ձ�NH3��������ˮ��������Ӧ���ȣ�������ʹ������ˮ������NH3���ߣ�������IV����NH3�����գ���Ӧǰ���������
����ͬѧ��δ���ǵ������е�ˮ������CO2�Ľ��룬������ɷ�Ӧ��������������൱��NH3�ļ��٣����½��ƫ�ͣ��ʶ�Ӧ�ټ�һ��ͬ���ĸ���ܣ�
����⣺����1������1����Ӧ�������������������δ��Ӧ�������AlN�У�
�ʴ�Ϊ����������
����2����1��������AlN������������Һ��Ӧ����NaAlO2�����ų�NH3���䷴Ӧ����ʽΪ��AlN+NaOH+H2O=NaAlO2+NH3����
�ʴ�Ϊ��AlN+NaOH+H2O=NaAlO2+NH3����
������NH3�����ʵ���Ϊ
=0.15mo�����ݵ�Ԫ���غ��֪10.00g��Ʒ�к���AlN�����ʵ���Ϊ0.15mol������Ϊ0.15mol��41g/mol=6.15g������Ʒ�е�AlN����������Ϊ
��100%=61.5%��
�ʴ�Ϊ��61.5%��
��2����Ӧ�������CO2��CO��CO2��CO��CO2��O2�Ļ�����壬�������������ܶȿɼ������������ƽ����Է�������Ϊ30��������������ض���CO2��CO������壮���������к���CO2xmol��CO ymol��������ԭ���غ���2x+y=0.18���٣�����ƽ����Է���������44x+28y=30��x+y�����ڣ��������̽�ã�x=0.02��y=0.14�����ԣ���Ʒ��C������Ϊ����0.02+0.14����12=1.92g��
�ʴ�Ϊ��1.92��
����3����1�����װ�õ��������������ڼ�ҩƷǰ���У����������ҩƷ���˷ѣ����ȼ��������ԣ�Ȼ������ƿ�з���������AlN��Ʒ���ٴӷ�Һ©������ƿ�м��������ŨNaOH���ⶨ�ռ���ˮ�������
�ʴ�Ϊ���ۢ٢ڢܣ�
��2����������ԵĹؼ���������ϵҪ�����ܱ�״̬�����õķ����У��ܷ������������������������Һ�������γ�Һ��߶Ȳ��װ�ÿ�֪���ʺ��������������������������Ϊ���رշ�Һ©������������ƿ�����ƿ���Ҳർ��ˮ������������ʱˮ���������䣮
�ʴ�Ϊ���رշ�Һ©������������ƿ�����ƿ���Ҳർ��ˮ������������ʱˮ���������䣮
��3��A�����ӷ������˷��Լ���Ӱ�찱������ⶨ����A�����ã�
B���ƾ��ӷ����ӷ������������ʵ����Ӱ�죬ͬʱ���ھƾ�������ˮ��Ҳ���ܴﵽ�����Ŀ�ģ���B����
C��ֲ���ͼȲ�����ˮ���ܶ�С��ˮҲ���ӷ������Ѱ�����ˮ���и��룬��C��ã�
D��CCl4�ܶȴ���ˮ�������������ã���D����
�ʴ�Ϊ��C��
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬�����ų�װ�õ����壬��ռ��ԭ������ռ䣬���ƿ�ڵ�Һ���Ƿ������Ӱ�����ռ�����NH3�����С��
�ʴ�Ϊ�����䣮
��5�����������ΪaL������£������ʵ���Ϊ
=
mol���ɷ���ʽAlN+NaOH+H2O=NaAlO2+NH3����֪����Ʒ��AlN�����ʵ���Ϊ=
mol������AlN������Ϊ
mol��41g/mol=
g����Ʒ��AIN����������Ϊ
��100%=
%��
�ʴ�Ϊ��
%��
��6������AlN�е����ʿ�����̼��Al2O3����ֻ̼������NaOH��Һ��Ӧ����ʱ���ܹ���Ϊ̼����Ʒ�к��е�������C��
ֻҪ֪��̼�������백������������ݰ����������AlN����������̼��AlN������֮������Ʒ������ϵ������ȷ���Ƿ����������ʣ�
�ʴ�Ϊ��̼��̼��������
����4��II��NH3���ױ����գ�������������ͬʱ�����к���ˮ������Ӱ�찱�������IJⶨ�����Բ����У������ĸ���Ϊ����װ��֮������ʢ�м�ʯ�ҵĸ���ܣ��ձ����ܵ�ĩ�˽�һ���۵�©�������հ�����
�ʴ�Ϊ�������У�II��NH3���ױ����գ�������������ͬʱ�����к���ˮ������Ӱ�찱�������IJⶨ����װ��֮������ʢ�м�ʯ�ҵĸ���ܣ��ձ����ܵ�ĩ�˽�һ���۵�©�������հ�����
����5��ͨ���ⶨ��������������ʵ���������������������㵪��������������Ϊ��һ���ְ���������ƿ�У���Ӧ�����İ�����ȫ����ϡ�������գ����ʵ����AlN������������ƫС��
��ͬѧ��Ƶ�װ�â�Ϻ���������ͨ����Ӧǰ���������������ⶨAlN����������������ĸ�������������ձ�NH3��������ˮ��������Ӧ���ȣ�������ʹ������ˮ������NH3���ߣ�������IV����NH3�����գ���Ӧǰ���������
����ͬѧ��δ���ǵ������е�ˮ������CO2�Ľ��룬������ɷ�Ӧ��������������൱��NH3�ļ��٣����½��ƫ�ͣ��ʶ�Ӧ�ټ�һ��ͬ���ĸ���ܣ�
�ʴ�Ϊ����Ӧ�����İ��������ܱ���ȫ���գ�III���У� ƫ�ͣ� Ӧ�ٽ�һ����IVװ������ȫ��ͬ�ĸ���ܣ����ٽ�һ��װ�м�ʯ�ҵĸ����Ҳ�У���
�ʴ�Ϊ����������
����2����1��������AlN������������Һ��Ӧ����NaAlO2�����ų�NH3���䷴Ӧ����ʽΪ��AlN+NaOH+H2O=NaAlO2+NH3����
�ʴ�Ϊ��AlN+NaOH+H2O=NaAlO2+NH3����
������NH3�����ʵ���Ϊ
| 3.36L |
| 22.4L/mol |
| 6.15g |
| 10g |
�ʴ�Ϊ��61.5%��
��2����Ӧ�������CO2��CO��CO2��CO��CO2��O2�Ļ�����壬�������������ܶȿɼ������������ƽ����Է�������Ϊ30��������������ض���CO2��CO������壮���������к���CO2xmol��CO ymol��������ԭ���غ���2x+y=0.18���٣�����ƽ����Է���������44x+28y=30��x+y�����ڣ��������̽�ã�x=0.02��y=0.14�����ԣ���Ʒ��C������Ϊ����0.02+0.14����12=1.92g��
�ʴ�Ϊ��1.92��
����3����1�����װ�õ��������������ڼ�ҩƷǰ���У����������ҩƷ���˷ѣ����ȼ��������ԣ�Ȼ������ƿ�з���������AlN��Ʒ���ٴӷ�Һ©������ƿ�м��������ŨNaOH���ⶨ�ռ���ˮ�������
�ʴ�Ϊ���ۢ٢ڢܣ�
��2����������ԵĹؼ���������ϵҪ�����ܱ�״̬�����õķ����У��ܷ������������������������Һ�������γ�Һ��߶Ȳ��װ�ÿ�֪���ʺ��������������������������Ϊ���رշ�Һ©������������ƿ�����ƿ���Ҳർ��ˮ������������ʱˮ���������䣮
�ʴ�Ϊ���رշ�Һ©������������ƿ�����ƿ���Ҳർ��ˮ������������ʱˮ���������䣮
��3��A�����ӷ������˷��Լ���Ӱ�찱������ⶨ����A�����ã�
B���ƾ��ӷ����ӷ������������ʵ����Ӱ�죬ͬʱ���ھƾ�������ˮ��Ҳ���ܴﵽ�����Ŀ�ģ���B����
C��ֲ���ͼȲ�����ˮ���ܶ�С��ˮҲ���ӷ������Ѱ�����ˮ���и��룬��C��ã�
D��CCl4�ܶȴ���ˮ�������������ã���D����
�ʴ�Ϊ��C��
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬�����ų�װ�õ����壬��ռ��ԭ������ռ䣬���ƿ�ڵ�Һ���Ƿ������Ӱ�����ռ�����NH3�����С��
�ʴ�Ϊ�����䣮
��5�����������ΪaL������£������ʵ���Ϊ
| aL |
| 22.4L/mol |
| a |
| 22.4 |
| a |
| 22.4 |
| a |
| 22.4 |
| 41a |
| 22.4 |
| ||
| wg |
| 4100a |
| 22.4w |
�ʴ�Ϊ��
| 4100a |
| 22.4w |
��6������AlN�е����ʿ�����̼��Al2O3����ֻ̼������NaOH��Һ��Ӧ����ʱ���ܹ���Ϊ̼����Ʒ�к��е�������C��
ֻҪ֪��̼�������백������������ݰ����������AlN����������̼��AlN������֮������Ʒ������ϵ������ȷ���Ƿ����������ʣ�
�ʴ�Ϊ��̼��̼��������
����4��II��NH3���ױ����գ�������������ͬʱ�����к���ˮ������Ӱ�찱�������IJⶨ�����Բ����У������ĸ���Ϊ����װ��֮������ʢ�м�ʯ�ҵĸ���ܣ��ձ����ܵ�ĩ�˽�һ���۵�©�������հ�����
�ʴ�Ϊ�������У�II��NH3���ױ����գ�������������ͬʱ�����к���ˮ������Ӱ�찱�������IJⶨ����װ��֮������ʢ�м�ʯ�ҵĸ���ܣ��ձ����ܵ�ĩ�˽�һ���۵�©�������հ�����
����5��ͨ���ⶨ��������������ʵ���������������������㵪��������������Ϊ��һ���ְ���������ƿ�У���Ӧ�����İ�����ȫ����ϡ�������գ����ʵ����AlN������������ƫС��
��ͬѧ��Ƶ�װ�â�Ϻ���������ͨ����Ӧǰ���������������ⶨAlN����������������ĸ�������������ձ�NH3��������ˮ��������Ӧ���ȣ�������ʹ������ˮ������NH3���ߣ�������IV����NH3�����գ���Ӧǰ���������
����ͬѧ��δ���ǵ������е�ˮ������CO2�Ľ��룬������ɷ�Ӧ��������������൱��NH3�ļ��٣����½��ƫ�ͣ��ʶ�Ӧ�ټ�һ��ͬ���ĸ���ܣ�
�ʴ�Ϊ����Ӧ�����İ��������ܱ���ȫ���գ�III���У� ƫ�ͣ� Ӧ�ٽ�һ����IVװ������ȫ��ͬ�ĸ���ܣ����ٽ�һ��װ�м�ʯ�ҵĸ����Ҳ�У���
����������Ϊ���͵���Ʒ���Ȳ������⣬��Ҫ������е�ʵ�鷽�������ۣ���ָ�����������е�ԭ���Լ�������ʵ��Ļ����Ͻ����µ�ʵ����ƣ�����ʵ��ԭ���ǹؼ����Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������ѧϰ��ȫ����ջ���֪ʶ���ѶȺܴ�

��ϰ��ϵ�д�
�����Ŀ
 ��2011?��ɽ��ģ�⣩��������ȼ�ϵ�ع���ʱ�ɲ����������⣨��ͼ1����������̼��Carbon����������С�Բ������������Ũ�ȣ�concentration����Ӱ�죨��Ӱ��������ͼ2��ʾ����
��2011?��ɽ��ģ�⣩��������ȼ�ϵ�ع���ʱ�ɲ����������⣨��ͼ1����������̼��Carbon����������С�Բ������������Ũ�ȣ�concentration����Ӱ�죨��Ӱ��������ͼ2��ʾ����

 NH4++NH2-
NH4++NH2- ��2��¯�����Ƶ������ǽ���SO2���г�����ˮϴ��������¯����SO2�Ĵ��������ڽӴ����н��У�
��2��¯�����Ƶ������ǽ���SO2���г�����ˮϴ��������¯����SO2�Ĵ��������ڽӴ����н��У�