��Ŀ����
��ʽ̼��ͭ�ɱ�ʾΪ��xCuCO3��yCu(OH) 2��zH2O,�ⶨ��ʽ̼��ͭ��ɵķ����ж��֡�
![]() ��1���ֲ���������ԭ������ش��������⣺
��1���ֲ���������ԭ������ش��������⣺![]()
![]()
![]()
![]()
![]() ��д��xCuCO3��yCu(OH) 2��zH2O��������Ӧ�Ļ�ѧ����ʽ ��
��д��xCuCO3��yCu(OH) 2��zH2O��������Ӧ�Ļ�ѧ����ʽ ��
![]() ������װ�������������������Ӷ��ɣ������������������˳���ǣ����������ӿ���ĸ��ţ���
������װ�������������������Ӷ��ɣ������������������˳���ǣ����������ӿ���ĸ��ţ���
![]() ��a������ ���� ������ ���� ������ ���� ������ ���� ������ ���� ������l��
��a������ ���� ������ ���� ������ ���� ������ ���� ������ ���� ������l��
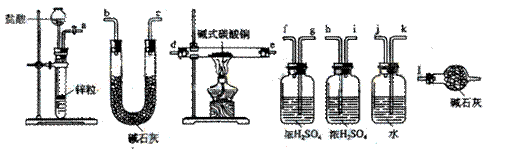 |
![]()
![]()
![]()
![]()
![]() �۳�ȡ23.9gij��ʽ̼��ͭ��Ʒ����ַ�Ӧ��õ�12.7g���������4.4g������̼��7.2gˮ������Ʒ�Ľᾧˮ����Ϊ g����ѧʽΪ ��
�۳�ȡ23.9gij��ʽ̼��ͭ��Ʒ����ַ�Ӧ��õ�12.7g���������4.4g������̼��7.2gˮ������Ʒ�Ľᾧˮ����Ϊ g����ѧʽΪ ��
![]() ��2��ijͬѧ�Ե���������������������ȫ�����������ⶨ��ʽ̼��ͭ����ɣ�����Ϊ�Ƿ���У���˵�����ɡ� ��
��2��ijͬѧ�Ե���������������������ȫ�����������ⶨ��ʽ̼��ͭ����ɣ�����Ϊ�Ƿ���У���˵�����ɡ� ��
(1)��xCuCO3��yCu(OH)2��zH2O+(x+y)H 2 = (x+y)Cu+ xCO2+(x+2y+z)H2O
![]() ��a��k,j��gf(hi)��de(ed)��hi(gf)��bc(cb)��l
��a��k,j��gf(hi)��de(ed)��hi(gf)��bc(cb)��l
![]() ��1.8 CuCO3��Cu(OH) 2��H2O
��1.8 CuCO3��Cu(OH) 2��H2O
![]() (2)���� ���ݷ�ӦxCuCO3��yCu(OH) 2��zH2O=(x+y)CuO+ xCO2��+(y+z)H2O��,���ݼ�ʽ̼��ͭ��CuO��CO2��H2O�����������������������������ɼ��������ɡ�
(2)���� ���ݷ�ӦxCuCO3��yCu(OH) 2��zH2O=(x+y)CuO+ xCO2��+(y+z)H2O��,���ݼ�ʽ̼��ͭ��CuO��CO2��H2O�����������������������������ɼ��������ɡ�
����:
��1������ļ�ʽ̼��ͭ��������Ӧ����һ�����ѵ���Ϣ����ʵϸ��һ��ֻҪ��������ΪCuCO3��Cu(OH)2���ȷֽ�����CuO����������Ӧ���ڢ��������Խ�������ڢ�Ҫ�ܷ������ⶨ��Ӧ��CO2��H2O��������˶���������������������ѡ���dz��Ȼ����ˮ��������ֹ�Ժ����ⶨӰ��Ϳ����ˣ���Ϊ�ⶨH2O��CO2�ֱ���Ũ����ͼ�ʯ���ǹ̶��ġ�
![]() ��2����ʵ�ڷ�����1���ٷ���ʽ��дʱ��õ��˼�ʽ̼��ͭ�ȷֽⷽ��ʽ�� xCuCO3��yCu(OH) 2��zH2O=(x+y)CuO+ xCO2��+(y+z)H2O�����Լӷ�����֪�����ݼ�ʽ̼��ͭ��CuO��CO2��H2O�����������������������������ɼ��������ɡ�
��2����ʵ�ڷ�����1���ٷ���ʽ��дʱ��õ��˼�ʽ̼��ͭ�ȷֽⷽ��ʽ�� xCuCO3��yCu(OH) 2��zH2O=(x+y)CuO+ xCO2��+(y+z)H2O�����Լӷ�����֪�����ݼ�ʽ̼��ͭ��CuO��CO2��H2O�����������������������������ɼ��������ɡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�

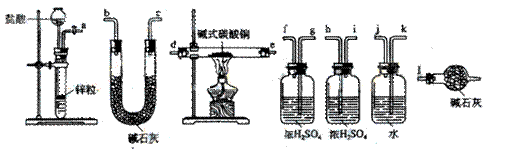
 ��1���ֲ���������ԭ������ش��������⣺
��1���ֲ���������ԭ������ش��������⣺