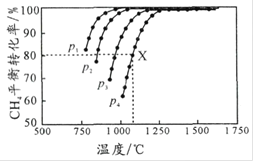
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΡ≥Μ·―ß Β―ι–ΓΉι¥” –≥Γ…œ¬ρά¥“ΜΤΩΡ≥ΤΖ≈Τ ≥”ΟΑΉ¥ΉΘ®÷ς“Σ «¥ΉΥαΒΡΥ°»ή“ΚΘ©Θ§”Ο Β―ι “±ξΉΦNaOH»ή“ΚΕ‘ΤδΫχ––ΒΈΕ®“‘≤βΕ®ΥϋΒΡΉΦ»Ζ≈®Ε»Θ§Άξ»ΪΖ¥”Π ±ΥυΒΟ»ή“ΚpH¥σ÷¬ΈΣ9 ΓΘœ¬±μ «4÷÷≥ΘΦϊ÷Η ΨΦΝΒΡ±δ…ΪΖΕΈßΘΚ
÷Η ΨΦΝ | ·»ο | ΦΉΜυ≥» | ΦΉΜυΚλ | Ζ”ΧΣ |
±δ…ΪΖΕΈßΘ®pHΘ© | 5.0ΓΪ8.0 | 3.1ΓΪ4.4 | 4.4ΓΪ6.2 | 8.2ΓΪ10.0 |
Θ®1Θ©ΗΟ Β―ι”Π―Γ”Ο Ής÷Η ΨΦΝΓΘ
Θ®2Θ©œ¬ΆΦ±μ Ψ50mLΒΈΕ®Ιή÷–“ΚΟφΒΡΈΜ÷ΟΘ§»τA”κCΩΧΕ»Φδœύ≤ν1mLΘ§A¥ΠΒΡΩΧΕ»ΈΣ25Θ§ΒΈΕ®Ιή÷–“ΚΟφΕΝ ΐ”ΠΈΣ mLΘΜΖ¥”Π¥οΒΈΕ®÷’Βψ ±ΒΡœ÷œσΈΣ ΓΘ

Θ®3Θ©ΈΣΦθ–Γ Β―ιΈσ≤νΘ§ΗΟΆ§―ß“ΜΙ≤Ϋχ––ΝΥ»ΐ¥Έ Β―ιΘ§ΦΌ…ηΟΩ¥ΈΥυ»ΓΑΉ¥ΉΧεΜΐΨυΈΣVmLΘ§NaOH±ξΉΦ“Κ≈®Ε»ΈΣc mo1/LΘ§»ΐ¥Έ Β―ιΫαΙϊΦ«¬Φ»γœ¬ΘΚ
Β―ι¥Έ ΐ | ΒΎ“Μ¥Έ | ΒΎΕΰ¥Έ | ΒΎ»ΐ¥Έ |
œϊΚΡNaOH»ή“ΚΧεΜΐ/mL | 26.02 | 25.35 | 25.30 |
¥”…œ±μΩ…“‘Ω¥≥ωΘ§ΒΎ“Μ¥Έ Β―ι÷–Φ«¬ΦœϊΚΡNaOH»ή“ΚΒΡΧεΜΐΟςœ‘Εύ”ΎΚσΝΫ¥ΈΘ§Τδ‘≠“ρΩ…Ρή « ΓΘ
AΘ° Β―ιΫα χ ±Θ§Η© ”ΩΧΕ»œΏΕΝ»ΓΒΈΕ®÷’Βψ ±NaOH»ή“ΚΒΡΧεΜΐΘΜ
BΘ°ΒΈΕ®«ΑΒΈΕ®ΙήΦβΉλ”–Τχ≈ίΘ§ΒΈΕ®Ϋα χΦβΉλ≤ΩΖ÷≥δ¬ζ»ή“ΚΘΜ
CΘ° ΔΉΑΑΉ¥Ή»ή“ΚΒΡΒΈΕ®Ιή”Ο’τΝσΥ°œ¥ΙΐΘ§Έ¥”ΟΑΉ¥Ή»ή“Κ»σœ¥ΘΜ
DΘ°ΉΕ–ΈΤΩ‘Λœ»”Ο ≥”ΟΑΉ¥Ή»σœ¥ΙΐΘΜ
EΘ°ΒΈΦ”NaOH»ή“Κ ±Θ§Έ¥≥δΖ÷’ώΒ¥Θ§Η’Ω¥ΒΫ»ή“Κ±δ…ΪΘ§ΝΔΩΧΆΘ÷ΙΒΈΕ®ΓΘ
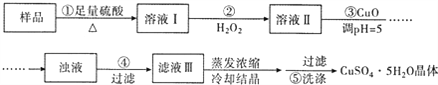
Θ®4Θ©ΗυΨίΥυΗχ ΐΨίΘ§–¥≥ωΦΤΥψΗΟΑΉ¥Ή÷–¥ΉΥαΒΡΈο÷ ΒΡΝΩ≈®Ε»ΒΡ±μ¥ο Ϋ(≤Μ±ΊΜ·Φρ)ΘΚCΘΫ mol/LΓΘ
ΓΨ¥πΑΗΓΩΘ®1Θ©Ζ”ΧΣ
Θ®2Θ©25.40ΘΜ»ή“Κ¥”Έό…Ϊ±δΈΣΖέΚλ…ΪΘ§«“ΑκΖ÷÷”ΡΎ≤ΜΆ …Ϊ
Θ®3Θ©BD
Θ®4Θ©![]()
ΓΨΫβΈωΓΩ
Θ®1Θ© ≥¥Ή÷–ΒΡ¥ΉΥα «»θΥαΘ§«β―θΜ·ΡΤ ««ΩΦνΘ§Υυ“‘ΒΈΕ®ΚσΒΡ»ή“Κ≥ Φν–‘Θ§Υυ“‘―Γ‘ώΖ”ΧΣΉς÷Η ΨΦΝΘ§Ι ¥πΑΗΈΣΘΚΖ”ΧΣΘΜ
Θ®2Θ©A”κCΩΧΕ»Φδœύ≤ν1mLΘ§ΟΩ“Μ–ΓΗώ «0.1mlΘ§ΒΈΕ®ΙήΒΡΕΝ ΐΨΪ»ΖΒΫ0.01mLΘ§Υυ“‘’ΐ»ΖΕΝ ΐ «25.40mLΘΜΖ”ΧΣ”ωΥα≤Μ±δ…ΪΘ§Υυ“‘»ή“ΚΩΣ ΦΈό…ΪΘ§ΒΈΕ®÷’Βψ ±ΈΣ»θΦν–‘»ή“ΚΘ§Υυ“‘÷’Βψ ±ΒΡœ÷œσ «»ή“Κ”…Έό…Ϊ±δΈΣ«≥Κλ…ΪΜρΖέΚλ…ΪΘ§«“ΑκΖ÷÷”ΡΎ≤ΜΆ …ΪΘ§Ι ¥πΑΗΈΣΘΚ25.40ΘΜ»ή“Κ¥”Έό…Ϊ±δΈΣΖέΚλ…ΪΘ§«“ΑκΖ÷÷”ΡΎ≤ΜΆ …ΪΘΜ
Θ®3Θ©AΓΔ Β―ιΫα χ ±Θ§Η© ”ΩΧΕ»œΏΕΝ»ΓΒΈΕ®÷’Βψ ±NaOH»ή“ΚΒΡΧεΜΐΘ§Μα ΙΕΝ ΐΤΪ–ΓΘ§ΦΤΥψΒΡΧεΜΐ“≤ΤΪ–ΓΘ§Ι ¥μΈσΘΜ
BΓΔΒΈΕ®«Α”–Τχ≈ίΘ§Μα Ι≥θΕΝ ΐΤΪ–ΓΘ§ΒΈΕ®Κσœϊ ßΘ§ΕΝ ΐ’ΐ»ΖΘ§‘ρΕΰ’ΏΒΡ≤ν÷ΒΤΪ¥σΘ§Ι ’ΐ»ΖΘΜCΓΔ ΔΉΑΑΉ¥Ή»ή“ΚΒΡΒΈΕ®Ιή”Ο’τΝσΥ°œ¥ΙΐΘ§Έ¥”ΟΑΉ¥Ή»ή“Κ»σœ¥Θ§Ε‘ΫαΙϊΈό”ΑœλΘ§Ι ¥μΈσΘΜ
DΓΔΉΕ–ΈΤΩ”Ο ≥¥Ή»σœ¥Θ§ Ι¥ΉΥαΒΡΈο÷ ΒΡΝΩ‘ωΕύΘ§œϊΚΡ«β―θΜ·ΡΤΒΡΧεΜΐ‘ω¥σΘ§ΫαΙϊΤΪ¥σΘ§Ι ’ΐ»ΖΘΜ
EΓΔΒΈΦ”NaOH»ή“Κ ±Θ§Έ¥≥δΖ÷’ώΒ¥Θ§Η’Ω¥ΒΫ»ή“Κ±δ…ΪΘ§ΝΔΩΧΆΘ÷ΙΒΈΕ®Θ§ΒΈΕ®÷’ΒψΜΙΈ¥ΒΫ¥οΘ§ ΙΫαΙϊΤΪ–ΓΘ§Ι ¥μΈσΘΜ
BD’ΐ»ΖΘ§Ι ¥πΑΗΈΣΘΚBDΘΜ
Θ®4Θ©ΒΎ“Μ¥ΈΒΡ ΐΨί”κΚσΝΫ¥ΈΒΡ ΐΨίΟςœ‘¥σΘ§Υυ“‘…α»ΞΒΎ“Μ¥ΈΒΡ ΐΨίΘ§”ΟΚσΝΫ¥ΈΒΡ ΐΨίΫχ––ΦΤΥψΘ§ΗυΨί÷–ΚΆΖ¥”ΠΒΡ Β÷ Θ§¥ΉΥαΒΡ≈®Ε»ΈΣ![]() Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ![]() ΓΘ
ΓΘ

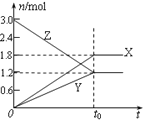
ΓΨΧβΡΩΓΩ7000C ±Θ§œρ»ίΜΐΈΣ2LΒΡΟή±’»ίΤς÷–≥δ»κ“ΜΕ®ΝΩΒΡCOΚΆH2OΘ§ΖΔ…ζΖ¥”ΠΘΚCO(g)+H2O(g)![]() CO2(g)+H2(g)ΘΜΖ¥”ΠΙΐ≥Χ÷–≤βΕ®ΒΡ≤ΩΖ÷ ΐΨίΦϊœ¬±μ(±μ÷–t2ΘΨt1)
CO2(g)+H2(g)ΘΜΖ¥”ΠΙΐ≥Χ÷–≤βΕ®ΒΡ≤ΩΖ÷ ΐΨίΦϊœ¬±μ(±μ÷–t2ΘΨt1)
Ζ¥”Π ±Φδ/min | n(CO)/mol | n(H2O)/mol |
0 | 1.20 | 0.60 |
t1 | 0.80 | |
t2 | 0.20 |
œ¬Ν–ΥΒΖ®÷–’ΐ»ΖΒΡ «
A.Ζ¥”Π‘Ύt1minΡΎΒΡΤΫΨυΥΌ¬ ΈΣΠ‘(CO2)=![]() molΓΛL-1ΓΛmin-1
molΓΛL-1ΓΛmin-1
B.ΒΫt1min ±Θ§Ζ¥”ΠΈ¥¥οΤΫΚβΉ¥Χ§
C.±Θ≥÷7000C≤Μ±δΘ§œρΤΫΚβΧεœΒ÷–‘ΌΆ®»κ0.60molCOΚΆ0.30 molH2OΘ§¥οΒΫ–¬ΤΫΚβ ±”κ‘≠ΤΫΚβœύ±»Θ§COΉΣΜ·¬ ‘ω¥σΘ§H2ΒΡΧεΜΐΖ÷ ΐ‘ω¥σ
D.Έ¬Ε»…ΐ÷Ν8000C ±Θ§…œ ωΖ¥”ΠΒΡΤΫΚβ≥Θ ΐΈΣ0.64Θ§‘ρ’ΐΖ¥”ΠΈΣΖ≈»»Ζ¥”Π