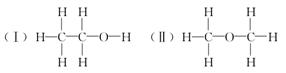��Ŀ����
ͨ����ʳ���Ϳɻ��ij�����л�������X,����Է�������Ϊ46,����̼����������Ϊ52.2%,�����������Ϊ13.0%��
(1)X�ķ���ʽ������������
(2)X������Ʒ�Ӧ�ų�����,��Ӧ�Ļ�ѧ����ʽ������������
(3)X������е�������ͭ�������·�Ӧ����Y,Y�Ľṹ��ʽ������������
(4)X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ����������,X��Z��Ӧ������һ������ζ������W,��184 g X��120 g Z��Ӧ������106 g W,����÷�Ӧ�IJ���(Ҫ��д���������)��
(1)X�ķ���ʽ������������
(2)X������Ʒ�Ӧ�ų�����,��Ӧ�Ļ�ѧ����ʽ������������
(3)X������е�������ͭ�������·�Ӧ����Y,Y�Ľṹ��ʽ������������
(4)X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ����������,X��Z��Ӧ������һ������ζ������W,��184 g X��120 g Z��Ӧ������106 g W,����÷�Ӧ�IJ���(Ҫ��д���������)��
(1)C2H6O(2��)
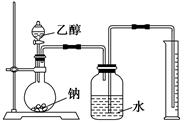
(2)2Na+2CH3CH2OH 2CH3CH2ONa+H2��(3��)
2CH3CH2ONa+H2��(3��)
(3)CH3CHO(2��)
(4)60.2%(3��)
(2)2Na+2CH3CH2OH
 2CH3CH2ONa+H2��(3��)
2CH3CH2ONa+H2��(3��)(3)CH3CHO(2��)
(4)60.2%(3��)
(1)��������,X�ķ����к�̼ԭ����Ϊ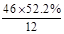 ��2,����ԭ����Ϊ
��2,����ԭ����Ϊ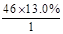 ��6,����ԭ����Ϊ
��6,����ԭ����Ϊ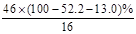 ��1,����X�ķ���ʽΪC2H6O��
��1,����X�ķ���ʽΪC2H6O��
(2)������⼰����������֪X���Ҵ����Ҵ����Ʒ�Ӧ�Ļ�ѧ����ʽΪ2Na+2CH3CH2OH 2CH3CH2ONa+H2����
2CH3CH2ONa+H2����
(3)X(�Ҵ�)������е�O2��ͭ�����������������·�Ӧ����Y(��ȩ),��ṹ��ʽΪCH3CHO��
(4)X(�Ҵ�)�����Ը��������Һ��Ӧ������Z(����)���������Ҵ���Ӧ,������������(W),���ݻ�ѧ����ʽ:CH3COOH+CH3CH2OH CH3COOC2H5+H2O,��֪184 g�Ҵ�����,Ӧ��120 g Z�������õ���������,�������Ͽ���������������������Ϊa������
CH3COOC2H5+H2O,��֪184 g�Ҵ�����,Ӧ��120 g Z�������õ���������,�������Ͽ���������������������Ϊa������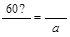 ,a="176" g�����Ը÷�Ӧ�IJ���Ϊ
,a="176" g�����Ը÷�Ӧ�IJ���Ϊ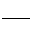 ��100%=60.2%��
��100%=60.2%��
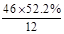 ��2,����ԭ����Ϊ
��2,����ԭ����Ϊ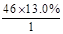 ��6,����ԭ����Ϊ
��6,����ԭ����Ϊ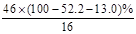 ��1,����X�ķ���ʽΪC2H6O��
��1,����X�ķ���ʽΪC2H6O��(2)������⼰����������֪X���Ҵ����Ҵ����Ʒ�Ӧ�Ļ�ѧ����ʽΪ2Na+2CH3CH2OH
 2CH3CH2ONa+H2����
2CH3CH2ONa+H2����(3)X(�Ҵ�)������е�O2��ͭ�����������������·�Ӧ����Y(��ȩ),��ṹ��ʽΪCH3CHO��
(4)X(�Ҵ�)�����Ը��������Һ��Ӧ������Z(����)���������Ҵ���Ӧ,������������(W),���ݻ�ѧ����ʽ:CH3COOH+CH3CH2OH
 CH3COOC2H5+H2O,��֪184 g�Ҵ�����,Ӧ��120 g Z�������õ���������,�������Ͽ���������������������Ϊa������
CH3COOC2H5+H2O,��֪184 g�Ҵ�����,Ӧ��120 g Z�������õ���������,�������Ͽ���������������������Ϊa������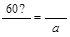 ,a="176" g�����Ը÷�Ӧ�IJ���Ϊ
,a="176" g�����Ը÷�Ӧ�IJ���Ϊ ��100%=60.2%��
��100%=60.2%��
��ϰ��ϵ�д�
�����Ŀ