��Ŀ����
��֪H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ?mol-1���ش��й��кͷ�Ӧ�����⣮
��1����0.1molBa��OH��2���ϡ��Һ������ϡ���ᷴӦ���ܷų�______kJ������
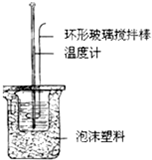
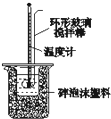
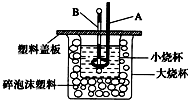
��2����ͼװ��������A��������______������ĭ���ϵ�������______��
��3����ͨ��ʵ��ⶨ�к��ȵġ�H��������������-57.3kJ?mol-1����ԭ�������______��
��4������ͬŨ�Ⱥ�����İ�ˮ��NH3?H2O������NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��______���ƫ����ƫС��������Ӱ�족����
��1����0.1molBa��OH��2���ϡ��Һ������ϡ���ᷴӦ���ܷų�______kJ������
��2����ͼװ��������A��������______������ĭ���ϵ�������______��
��3����ͨ��ʵ��ⶨ�к��ȵġ�H��������������-57.3kJ?mol-1����ԭ�������______��
��4������ͬŨ�Ⱥ�����İ�ˮ��NH3?H2O������NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��______���ƫ����ƫС��������Ӱ�족����

��1����H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ?mol-1��֪����1molH2O�ų�����Ϊ57.3kJ����0.1molBa��OH��2���ϡ��Һ������ϡ���ᷴӦ�ɵ�0.2molH2O�����Էų�������Ϊ57.3kJ��0.2=11.46kJ��
�ʴ�Ϊ��11.46��
��2������A�������ǻ��β�����������к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮����������ĭ���ϵ������ǣ����β�������������ȣ���������ɢʧ��
�ʴ�Ϊ�����β�������������ȣ���������ɢʧ��
��3��������Ч�����ã�������ɢʧ����õ��к�����ֵ�����С����H����-57.3kJ?mol-1��
�ʴ�Ϊ��ʵ�����������������ɢʧ��
��4����ˮΪ����������Ϊ���ȹ��̣�����ͬŨ�Ⱥ�����İ�ˮ��NH3?H2O������NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵƫС���ʴ�Ϊ��ƫС��
�ʴ�Ϊ��11.46��
��2������A�������ǻ��β�����������к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮����������ĭ���ϵ������ǣ����β�������������ȣ���������ɢʧ��
�ʴ�Ϊ�����β�������������ȣ���������ɢʧ��
��3��������Ч�����ã�������ɢʧ����õ��к�����ֵ�����С����H����-57.3kJ?mol-1��
�ʴ�Ϊ��ʵ�����������������ɢʧ��
��4����ˮΪ����������Ϊ���ȹ��̣�����ͬŨ�Ⱥ�����İ�ˮ��NH3?H2O������NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵƫС���ʴ�Ϊ��ƫС��

��ϰ��ϵ�д�
�����Ŀ