��Ŀ����
����Ŀ��ͼ���й����ϸ����Ҫ������ĸ���ͼ���ش��������⣺
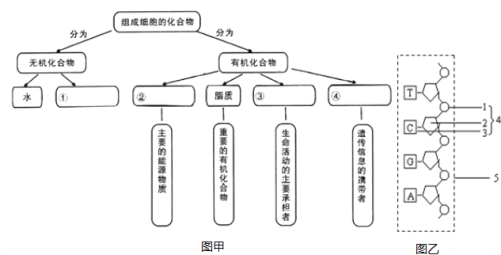
(1)д��ͼ���л����������:��____����____��
(2)ͼ���Т����Ԫ��Ϊ________________��
(3)ͼ��Ϊͼ���Тܵ�һ������,2��3�����Ʒֱ���____��______���ýṹ�����еļ��������______��(Ҫ��д����ȫ��)
(4)�������ڵĢ۵Ĺ�ͬ�Ļ�ѧԪ����_______����a�����������b����,���ij�����ʢۣ������ʢ���������ԭ�ӵĸ�����_____��
(5)SARS�����������ʢܳ���ˮ����������������ᡢ____��______��
���𰸡����� ������ C��H��O��N��P �������� ����� ������� C��H��O��N a+b ���� ���������A��G��C��U��
��������
1��������ͼ��֪�������Ƕ����ϸ���Ļ�������ۺ��Կ��飬���ϸ���Ļ��������������л������֣��������ˮ�����Σ��л���������ࡢ֬�ʡ������ʺͺ��ᣬ��������Ҫ����Դ���ʣ�֬�ʾ��ж��ֹ��ܣ������������������Ҫ�е��ߣ��������Ŵ���Ϣ��Я������2��DNA��������2������ƽ�е���������������ɵĹ����˫�����ṹ��������������ǽ���������������࣬���ɻ����Ǽܣ����λ���ڲ���ͨ����������γɼ���ԣ���RNA��ȣ�DNA�����еijɷ����������Ǻ�������़����
��1���ɷ�����֪��ͼ1�Т������Σ��������࣬���ǵ����ʣ����Ǻ�����
��2��ͼ���Т�Ϊ���ᣬ�����Ԫ��ΪC��H��O��N��P��
��3��������ͼ��֪��ͼ����һ��������������������1�����ᣬ2���������ǣ�3�ǰ���ण�4�ǰ��������������ýṹ�����еļ�������������
��4���������ڵĢ�Ϊ�����ʣ��乲ͬ�Ļ�ѧԪ����C��H��O��N����a�����������b��������ɵ����ʣ�����ÿ�����������ٺ���2��Oԭ�ӣ���a����������2a��Oԭ�ӣ�����ˮ�����γ����������У�ͨ����ȥH2O��ʧȥ�ˣ�a-b����O�����Ըõ�������������ԭ�ӵĸ�����2a-��a-b��=a+b��
��5��SARS�����������ʢ�ΪRNA������ˮ����������������������ǡ����������A��U��C��G����

����Ŀ��SO2�ķ��������ö��ڻ������������ش�ijС����ʵ�����ж�SO2�����ʼ��������õ�����������̽����
��1������ͼװ���Ʊ�������SO2������װ���з�Ӧ�Ļ�ѧ����ʽΪ________��װ�õ�����˳��Ϊ��a��________��������������Сд��ĸ��ʾ��

��2������ͼװ��̽��SO2�����ʡ���ѡ�Լ���NaOH��Һ��ϡH2SO4��H2O2��Һ��FeCl3��Һ�� ����-KI��Һ������H2S��Һ��

�������� | ʵ������ | ����ԭ�� |
��ע����������H2S��Һע�����SO2����ƿ | �������ɫ���� | +4��S��_______�� |
��ע������_______��Һע�����SO2����ƿ | _______ | +4��S�л�ԭ�ԣ���Ӧ�����ӷ���ʽΪ_______ |
��3����ҵ�ϻ�������SO2��һ��;���ǣ�
![]()
��С����ʵ����̽�������ʱ��һ����������100mL c0mol��L��1��(NH4)2SO3��Һͨ����������ⶨ��Һ��(NH4)2SO3��������(��)��
��Ϊ��С�����ʵ�鷽�������������������̵�ϸ�ڣ�����������ֵ����ĸ��ʾ����______________________________________________________________________________
�ڦ� =________��100%����ʵ�鷽���е���������ʾ����