��Ŀ����
ʵ����ģ���ù�ҵ�������壨����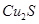 ��Al2O3��Fe2O3��SiO2�ȣ���ȡ��ͭ���̷���
��Al2O3��Fe2O3��SiO2�ȣ���ȡ��ͭ���̷���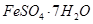 ����������Kal��SO4��2
����������Kal��SO4��2 12H2O�ݵIJ����������£�
12H2O�ݵIJ����������£�
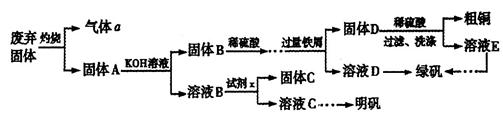
��1���Լ�x��_________��д��ѧʽ����
��2����ƽ���з���ʽ��
____
��4��Ϊ�˷�����Ʒ���̷�������Ԫ�صĺ�����ijͬѧ��ȡ20.0g��Ʒ���100mL��Һ����ȡ25.00mL��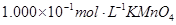 ����Һ���еζ���MnO
����Һ���еζ���MnO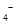 ����ԭΪ
����ԭΪ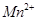 ����
����
��ش��������⣺
�ٵζ�ʱ��KmnO4����ҺӦʢ����______________�����������ƣ��С�
�����ﵽ�ζ��յ�����KmnO4����Һ���Ϊ25.00mL����ò�Ʒ����Ԫ�ص���������Ϊ_________��
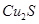 ��Al2O3��Fe2O3��SiO2�ȣ���ȡ��ͭ���̷���
��Al2O3��Fe2O3��SiO2�ȣ���ȡ��ͭ���̷���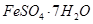 ����������Kal��SO4��2
����������Kal��SO4��2 12H2O�ݵIJ����������£�
12H2O�ݵIJ����������£�
��1���Լ�x��_________��д��ѧʽ����
��2����ƽ���з���ʽ��
____

��4��Ϊ�˷�����Ʒ���̷�������Ԫ�صĺ�����ijͬѧ��ȡ20.0g��Ʒ���100mL��Һ����ȡ25.00mL��
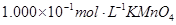 ����Һ���еζ���MnO
����Һ���еζ���MnO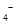 ����ԭΪ
����ԭΪ ����
������ش��������⣺
�ٵζ�ʱ��KmnO4����ҺӦʢ����______________�����������ƣ��С�
�����ﵽ�ζ��յ�����KmnO4����Һ���Ϊ25.00mL����ò�Ʒ����Ԫ�ص���������Ϊ_________��
��1��H2SO4����KHSO4����2�֣�
��2������Ũ���������Ũ������Ũ��������ȴ�ᾧ��4�֣�
��3��2��5��2��2��5��4��3�֣�
��4������ʽ�ζ��ܣ�2�֣� ��14%��3�֣�
��2������Ũ���������Ũ������Ũ��������ȴ�ᾧ��4�֣�
��3��2��5��2��2��5��4��3�֣�
��4������ʽ�ζ��ܣ�2�֣� ��14%��3�֣�
�����������1������KOH����ҺB����K[Al(OH)4],��������Ϊ����������XΪH2SO4����KHSO4����
��3�����ݻ��ϼ�������������ƽ��ѧ����ʽ��
��4���ٸ�����ؾ���ǿ�����ԣ��������ܣ�ֻ��ʢ������ʽ�ζ����У�
�ڸ��ݵ����غ��֪��KMnO4��5Fe2+,20.0��Ʒ�к�����0.025L��0.1mol/L��5��4��56g/mol=2.8g�����������Ԫ�ص�����������

��ϰ��ϵ�д�
�����Ŀ
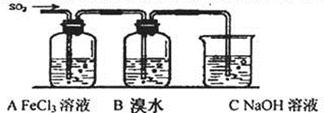


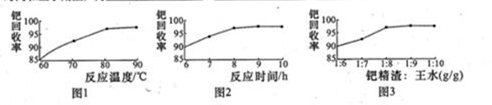
 N2 + CO2�����й��ڴ˷�Ӧ��˵���У�������ǣ� ��
N2 + CO2�����й��ڴ˷�Ӧ��˵���У�������ǣ� ��