��Ŀ����
��ҵ������������Ҫ�ɷ�ΪAl2O3������������Fe2O3��SiO2�����ʣ���ȡ��������ұ������ԭ�ϣ���ȡ�IJ����������£�
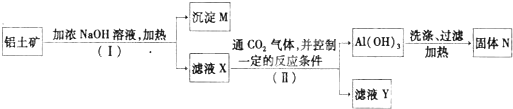
��1����͢����з�����Һ�ͳ����IJ����ǣ�
��2������M�г�������ɳ�⣬һ��������
��3����ҺX�У�����Ԫ�ص����ʵĻ�ѧʽΪ
��4��ʵ�����ﳣ��AICl3��Һ�м���

��1����͢����з�����Һ�ͳ����IJ����ǣ�
����
����
����
����
��2������M�г�������ɳ�⣬һ��������
Fe2O3
Fe2O3
������N��Al2O3
Al2O3
����3����ҺX�У�����Ԫ�ص����ʵĻ�ѧʽΪ
NaAlO2
NaAlO2
����������
��
����ᡱ��������Ρ��������ʣ�д���������������Ӧ�Ļ�ѧ����ʽAl2O3+2NaOH=2NaAlO2+H2O��SiO2+2NaOH=Na2SiO3+H2O
Al2O3+2NaOH=2NaAlO2+H2O��SiO2+2NaOH=Na2SiO3+H2O
����4��ʵ�����ﳣ��AICl3��Һ�м���
��ˮ
��ˮ
�����ˮ����NaOH��Һ��������ȡA1��OH��3��д���÷�Ӧ�Ļ�ѧ����ʽ3NH3?H2O+AlCl3=Al��OH��3+3NH4Cl
3NH3?H2O+AlCl3=Al��OH��3+3NH4Cl
��������Fe2O3���������Ʋ���Ӧ���������������������ܷ�Ӧ������I���ù��˳�ȥFe2O3������MΪ�������Ȳ������ҺX����ƫ�����ơ������ƣ�����IIͨ�������̼����������ʹ������ת��Ϊ�����������������˵��������������ȷֽ����������õ�����������ҺY���й����Ρ�̼���Σ�
��1�������������Һ�ķ���Ϊ���ˣ�
��2���ɹ������̿�֪��MΪFe2O3��NΪAl2O3��
��3����ҺX����ƫ�����ƣ������Σ������������������������������������Һ��Ӧ����ƫ�����ơ���������ˮ��
��4��������������ǿ�ʵ����ͨ�������ˮ�������Ʊ�����������������ˮ���Ȼ�����Ӧ���������������Ȼ�泥�
��1�������������Һ�ķ���Ϊ���ˣ�
��2���ɹ������̿�֪��MΪFe2O3��NΪAl2O3��
��3����ҺX����ƫ�����ƣ������Σ������������������������������������Һ��Ӧ����ƫ�����ơ���������ˮ��
��4��������������ǿ�ʵ����ͨ�������ˮ�������Ʊ�����������������ˮ���Ȼ�����Ӧ���������������Ȼ�泥�
����⣺Fe2O3���������Ʋ���Ӧ���������������������ܷ�Ӧ������I���ù��˳�ȥFe2O3������MΪ�������Ȳ������ҺX����ƫ�����ơ������ƣ�����IIͨ�������̼����������ʹ������ת��Ϊ�����������������˵��������������ȷֽ����������õ�����������ҺY���й����Ρ�̼���Σ�
��1��I��II��������������Һ�ķ���Ϊ���ˣ�
�ʴ�Ϊ�����ˣ����ˣ�
��2���ɹ������̿�֪��MΪFe2O3��NΪAl2O3��
�ʴ�Ϊ��Fe2O3��Al2O3��
��3���ɹ������̿�֪����ҺX�У�����Ԫ�ص����ʵĻ�ѧʽΪ��NaAlO2�������Σ��������������Ӧ�Ļ�ѧ����ʽΪ��Al2O3+2NaOH=2NaAlO2+H2O��SiO2+2NaOH=Na2SiO3+H2O��
�ʴ�Ϊ��NaAlO2���Σ�Al2O3+2NaOH=2NaAlO2+H2O��SiO2+2NaOH=Na2SiO3+H2O��
��4��������������ǿ�ʵ����ͨ�������ˮ�������Ʊ�����������������ˮ���Ȼ�����Ӧ���������������Ȼ�泥���Ӧ����ʽΪ��3NH3?H2O+AlCl3=Al��OH��3+3NH4Cl��
�ʴ�Ϊ����ˮ��3NH3?H2O+AlCl3=Al��OH��3+3NH4Cl��
��1��I��II��������������Һ�ķ���Ϊ���ˣ�
�ʴ�Ϊ�����ˣ����ˣ�
��2���ɹ������̿�֪��MΪFe2O3��NΪAl2O3��
�ʴ�Ϊ��Fe2O3��Al2O3��
��3���ɹ������̿�֪����ҺX�У�����Ԫ�ص����ʵĻ�ѧʽΪ��NaAlO2�������Σ��������������Ӧ�Ļ�ѧ����ʽΪ��Al2O3+2NaOH=2NaAlO2+H2O��SiO2+2NaOH=Na2SiO3+H2O��
�ʴ�Ϊ��NaAlO2���Σ�Al2O3+2NaOH=2NaAlO2+H2O��SiO2+2NaOH=Na2SiO3+H2O��
��4��������������ǿ�ʵ����ͨ�������ˮ�������Ʊ�����������������ˮ���Ȼ�����Ӧ���������������Ȼ�泥���Ӧ����ʽΪ��3NH3?H2O+AlCl3=Al��OH��3+3NH4Cl��
�ʴ�Ϊ����ˮ��3NH3?H2O+AlCl3=Al��OH��3+3NH4Cl��
�����������Թ�����������ʽ�������Ļ���������ʡ����ʵķ����ᴿ�����ڻ�ѧ������д�ȣ��Ѷ��еȣ������������ԭ���ǹؼ����Ƕ�֪ʶǨ�Ƶ��ۺ����ã�

��ϰ��ϵ�д�
 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
�����Ŀ



