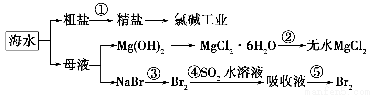��Ŀ����
���ú�ˮ������ȡ���þ����ȡ�������¡�
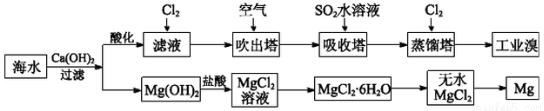
(1)��ȡ��Ĺ����У���������Br����Br2ת����Ŀ����________���������з�����Ӧ�����ӷ���ʽ��__________����ƽ���ƶ�ԭ������ͨ��������ҪĿ����_______��
(2)��MgCl2��Һ�еõ�MgCl2��6H2O�������Ҫ������________________�����ˡ�ϴ�ӡ����
(3)�����������̣�����10 m3��ˮ�е���Ԫ��ת��Ϊ��ҵ�壬������Ҫ��״����Cl2�����Ϊ________L(����Cl2���ܽ�)��
(1)����Ԫ�ؽ��и�����SO2��Br2��2H2O=4H����2Br����SO42����ͨ�������������������ʹBr2(aq) Br2(g)ƽ�������ƶ���(2)����Ũ������ȴ�ᾧ
Br2(g)ƽ�������ƶ���(2)����Ũ������ȴ�ᾧ
(3)179.2
��������(2)���ڵõ�����MgCl2��6H2O��������Ҫ��������Ũ������ȴ�ᾧ�����˵Ȳ��裬������ֱ�ӽ���Һ���ɡ�(3)10 m3��ˮ��Br��������Ϊ104L��64��10��3 g��L��1��640 g������Cl2��2Br��=2Cl����Br2����֪һ��ת������89.6 L Cl2(��״����)��������Ҫ179.2 L Cl2(��״����)��

��ϰ��ϵ�д�
�����Ŀ