题目内容
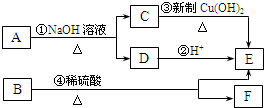
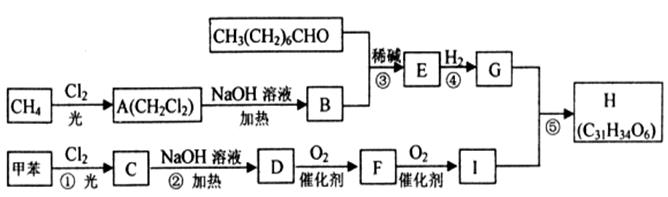
A、B两种有机物均是有机合成的中间体,其中A的分子式为C4H7O2Br,B分子中含2个氧原子,其燃烧产物n(CO2):n(H2O)=2:1,质谱图表明B的相对分子质量为188。A和B存在如下转化关系:
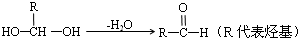
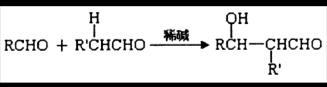
已知:①一个碳原子上连有两个羟基时,易发生下列转化:
②同一个碳原子上连有两个双键的结构不稳定。
请回答:
(1)C跟新制的氢氧化铜反应的化学方程式是 。
(2)A的结构简式是 。
(3)B的分子式是 。
(4)F具有如下特点:①具有弱酸性;②核磁共振氢谱中显示五种吸收峰;③苯环上的一氯代物只有两种;④除苯环外,不含有其他环状结构。写出符合上述条件且具有稳定结构的任意两种同分异构体的结构简式: 、 。

已知:①一个碳原子上连有两个羟基时,易发生下列转化:

②同一个碳原子上连有两个双键的结构不稳定。
请回答:
(1)C跟新制的氢氧化铜反应的化学方程式是 。
(2)A的结构简式是 。
(3)B的分子式是 。
(4)F具有如下特点:①具有弱酸性;②核磁共振氢谱中显示五种吸收峰;③苯环上的一氯代物只有两种;④除苯环外,不含有其他环状结构。写出符合上述条件且具有稳定结构的任意两种同分异构体的结构简式: 、 。
(1)CH3CHO+2Cu(OH)2 CH3COOH+Cu2O↓+2H2O(2分)
CH3COOH+Cu2O↓+2H2O(2分)
(2)CH3COOCHBrCH3(2分);(3)C12H12O2(1分)
(4)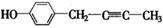 、
、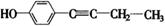 、
、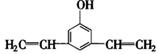 、
、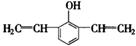 (任写两个,每个2分)
(任写两个,每个2分)
 CH3COOH+Cu2O↓+2H2O(2分)
CH3COOH+Cu2O↓+2H2O(2分)(2)CH3COOCHBrCH3(2分);(3)C12H12O2(1分)
(4)
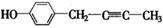 、
、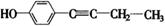 、
、 、
、 (任写两个,每个2分)
(任写两个,每个2分)试题分析:C、D都能转化成E,说明C、D含有的碳原子数相等,都含有2个碳原子,C能与新制备氢氧化铜反应,C为CH3CHO,E为CH3COOH,则D为CH3COONa,结合A的分子式以及题给信息可知A为CH3COOCHBrCH3,B分子中含2个氧原子,其燃烧产物n(CO2):n(H2O)=2:1,说明分子中C、H原子数相等,质谱图表明B的相对分子质量为188,设分子式为CnHnO2,则13n+32=188,n=12,则分子式为C12H12O2,则F的分子式为C10H10O,Ω=
 =6,
=6,(1)C为CH3CHO,与氢氧化铜反应的方程式为CH3CHO+2Cu(OH)2
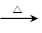 CH3COOH+Cu2O↓+2H2O;
CH3COOH+Cu2O↓+2H2O; (2)由以上分析可知A为CH3COOCHBrCH3;
(3)B的分子式是C12H12O2,;
(4)F的分子式为C10H10O,Ω=
 =6,①具有弱酸性,应含有酚羟基;
=6,①具有弱酸性,应含有酚羟基;②核磁共振氢谱中显示五种吸收峰;③苯环上的一氯代物只有两种,应为对称结构;④除苯环外,不含有其他环状结构,分子中应含有1个C≡C或2个C=C,则可能的结构为
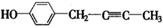 、
、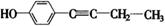 、
、 、
、

练习册系列答案
 黄冈冠军课课练系列答案
黄冈冠军课课练系列答案
相关题目
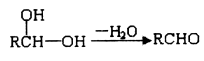
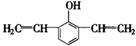
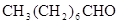 有多种同分异构体,符合下列性质的结构简式是:
有多种同分异构体,符合下列性质的结构简式是: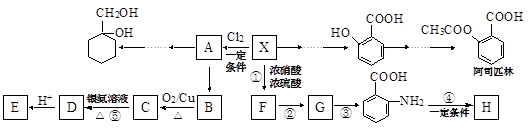
 (苯胺,易被氧化)
(苯胺,易被氧化)
 >H2CO3>
>H2CO3>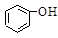 >HCO3―,向Na2CO3溶液中逐滴加入水杨酸(
>HCO3―,向Na2CO3溶液中逐滴加入水杨酸( )溶液,可能发生的反应的离子方程式书写正确的是
)溶液,可能发生的反应的离子方程式书写正确的是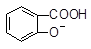 +HCO3―
+HCO3― +HCO3―
+HCO3― ,它既不溶于水,也不溶于碳酸氢钠溶液,能够跟1mol该化合物起反应的H2或Br2的最大用量分别是( )
,它既不溶于水,也不溶于碳酸氢钠溶液,能够跟1mol该化合物起反应的H2或Br2的最大用量分别是( )
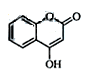 是一种医药中间体,常用来制备抗凝血药,可通过下列路线合成:
是一种医药中间体,常用来制备抗凝血药,可通过下列路线合成: