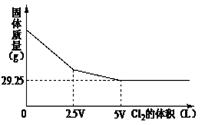
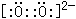��Ŀ����
��1�������͵�FeCl3��Һ��μ��뵽��ˮ�У���Һ����ɫ��ϼ���ɵõ�һ�ֺ��ɫ��Һ�塣д���ù��̵����ӷ���ʽ
��������Һ����μ���ϡ������Һ��������������
��Ӧ�����ӷ���ʽ
��2����Fe2����NO3����Fe3����NH4����H����H2O�������ӣ��ֱ�����ͬһ������ԭ��Ӧ�еķ�Ӧ����������÷�Ӧ�����ӷ���ʽ��
���л�ԭ���������������ʵ���֮��Ϊ ��
��������Һ����μ���ϡ������Һ��������������
��Ӧ�����ӷ���ʽ
��2����Fe2����NO3����Fe3����NH4����H����H2O�������ӣ��ֱ�����ͬһ������ԭ��Ӧ�еķ�Ӧ����������÷�Ӧ�����ӷ���ʽ��
���л�ԭ���������������ʵ���֮��Ϊ ��
(1)Fe3+ +3H2O  Fe(OH)3(����)+3H+ �������к��ɫ�������ɣ��ټ�����������ܽ����ӷ���ʽFe(OH)3+3H+=Fe3++3H2O
Fe(OH)3(����)+3H+ �������к��ɫ�������ɣ��ټ�����������ܽ����ӷ���ʽFe(OH)3+3H+=Fe3++3H2O
��2��8Fe2++NO3��+10H+==NH4++8Fe3++5H2O 8:1
 Fe(OH)3(����)+3H+ �������к��ɫ�������ɣ��ټ�����������ܽ����ӷ���ʽFe(OH)3+3H+=Fe3++3H2O
Fe(OH)3(����)+3H+ �������к��ɫ�������ɣ��ټ�����������ܽ����ӷ���ʽFe(OH)3+3H+=Fe3++3H2O ��2��8Fe2++NO3��+10H+==NH4++8Fe3++5H2O 8:1
��1������������Һ��ˮ�����������������壬����ʽΪFe3+ +3H2O  Fe(OH)3(����)+3H+���������ʹ����۳����������������ԣ����ܽ������������������������к��ɫ�������ɣ��ټ�����������ܽ⣬��Ӧ�����ӷ���ʽΪFe(OH)3+3H+=Fe3++3H2O��
Fe(OH)3(����)+3H+���������ʹ����۳����������������ԣ����ܽ������������������������к��ɫ�������ɣ��ټ�����������ܽ⣬��Ӧ�����ӷ���ʽΪFe(OH)3+3H+=Fe3++3H2O��
��2�����������������ǿ�������ӵģ������ڷ�Ӧ��Ӧ�������������������ӣ�����ʽΪ8Fe2++NO3��+10H+==NH4++8Fe3++5H2O���������е�Ԫ�صĻ��ϼ۴ӣ�5�۽��͵���3�ۣ��õ�8�����ӣ���ԭ��������������1�����ӣ����Ը��ݵ��ӵĵ�ʧ�غ��֪����ԭ���������������ʵ���֮����8:1��
 Fe(OH)3(����)+3H+���������ʹ����۳����������������ԣ����ܽ������������������������к��ɫ�������ɣ��ټ�����������ܽ⣬��Ӧ�����ӷ���ʽΪFe(OH)3+3H+=Fe3++3H2O��
Fe(OH)3(����)+3H+���������ʹ����۳����������������ԣ����ܽ������������������������к��ɫ�������ɣ��ټ�����������ܽ⣬��Ӧ�����ӷ���ʽΪFe(OH)3+3H+=Fe3++3H2O����2�����������������ǿ�������ӵģ������ڷ�Ӧ��Ӧ�������������������ӣ�����ʽΪ8Fe2++NO3��+10H+==NH4++8Fe3++5H2O���������е�Ԫ�صĻ��ϼ۴ӣ�5�۽��͵���3�ۣ��õ�8�����ӣ���ԭ��������������1�����ӣ����Ը��ݵ��ӵĵ�ʧ�غ��֪����ԭ���������������ʵ���֮����8:1��

��ϰ��ϵ�д�
 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�
�����Ŀ
 4H����2Fe2����________��
4H����2Fe2����________��