��Ŀ����
þ��һ�����ʻ��õĽ�������ش��������⣺
(1)����ͬѧͨ��ʵ��̽��Mg�ܷ���CO2������ȼ�գ���þ���ڿ����е�ȼ��Ѹ�ٲ���ʢ��CO2����ļ���ƿ�У��۲쵽þ������ȼ�գ�ƿ�ڱڳ��ֺ�ɫ�������������д��Mg��CO2��ȼ�յĻ�ѧ����ʽ��__________
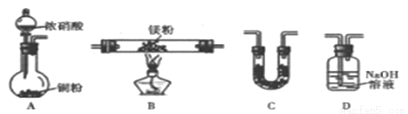
(2)����ͬѧ���ݼ���ʵ�飬�Ʋ�MgҲ����NO2��ȼ�գ����ܲ���ΪMgO ��Mg3N2����N2����ͨ����ͼ����װ�ü�ҩƷ����֤��Ӧ����г�װ��ʡ�ԣ������������ظ�ʹ�ã���
��֪��a. Mg3N2��ˮǿ��ˮ�⡣
b. NO2�����ܱ�NaOH���ա�
c.25��ʱ�����볣����CH3COOH��Ka=1.8��10-5 NH3��H2O��Ka=1.8��10-5
�ش��������⣺
������ͬѧʵ��װ�����ӵ���ȷ˳��ΪA��__________����װ����ĸ����װ��C��ʢ�ŵ��Լ���________��
�ڵ�ʵ������г���_________����ʱ�����ܿ�ʼ���ȣ�ȷ����������N2���ɵ�ʵ������Ϊ_________��
��װ��D�������������ʵ�����ȵ����Σ������ӷ���ʽΪ___________��
(3)���ʵ��֤����
�ٲ����д���Mg3N2��__________________��
��Mg(OH)2��NH4+ֱ�ӷ�Ӧ���ܡ�������
_____________��
��ϰ��ϵ�д�
�����Ŀ




 D����NH2
D����NH2