��Ŀ����
����Ŀ����һƿ�������Һ�����п��ܺ���NH![]() ��K����Mg2����Ba2����Al3����Fe3����SO42-��CO32-��NO3-��I����Cl����ȡ����Һ��������ʵ�飺
��K����Mg2����Ba2����Al3����Fe3����SO42-��CO32-��NO3-��I����Cl����ȡ����Һ��������ʵ�飺
����pH��ֽ��ø���Һ�����ԣ�
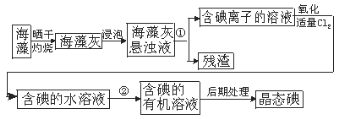
��ȡ������Һ�������������Ƶ���ˮ������CCl4���������ú�CCl4����Ϻ�ɫ��
����ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������Ϊ���ԣ��������μӹ������������ɣ�
��ȡ��������������Һ������Na2CO3��Һ���а�ɫ�������ɣ�
�ݽ��۵õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ����ʵ�ش����⣺
��1��д������������Ӧ�����ӷ���ʽ_________��
��2������Һ�п϶����ڵ�������________��
��3������Һ�п϶������ڵ�������________��
��4������Һ�л�����ȷ���Ƿ���ڵ�������_________��
���𰸡�Cl2��2I��=I2��2Cl�� NH4+��Ba2����I�� Mg2����Al3����Fe3����SO42-��CO32-��NO3- K����Cl��
��������
���ݳ�����Һ�ã�ԭ��Һû�����Ӧ�����ӣ�
�ٸ���ʵ��(1)��Һ�������ж�������һ�����ڣ��������ӷ�Ӧ�����Ӳ��ܹ����棻
�ڸ���ʵ��(2)��������CCl4������������ˮ������CCl4����Ϻ�ɫ��˵����Һ��һ�����е����ӣ��ܹ�������ӷ�Ӧ�����Ӳ����棻
�۸���ʵ��(3)����NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ���������жϷ��������������ӷ�Ӧ���ɳ��������Ӳ����ڣ�
�ܸ���ʵ��(4)��ȡ��������������Һ��Na2CO3��Һ���а�ɫ�������ɣ���֪һ������̼������ӣ��ų�������ӷ�Ӧ�����ӣ�
������ɫ��Ӧ���麬�еĽ��������ӡ�
�ٸ���ʵ��(1)������Һ��ǿ���ԣ�˵����Һ�п϶�����H+����H+��CO32-��Ӧ������Ӧ�����ܹ��棬˵����Һ�п϶�������CO32-��
�ڸ���ʵ��(2)����CCl4����Ϻ�ɫ��˵����I2����������I-�����������������ģ��Ӷ�˵����Һ�к���I-����I-��Fe3+��NO3-��H+�ܷ���������ԭ��Ӧ�������ܹ��棬˵����Һ�п϶�������Fe3+��NO3-��
�۸���ʵ��(3)������Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ��������������Fe3+��Mg2+��Al3+����Ӧ����������˵����Һ�п϶�������Fe3+��Mg2+��Al3+��
�ܸ���ʵ��(4)����ȡ����������������Һ��Na2CO3��Һ���а�ɫ�������ɣ�˵����Һ�п϶�����Ba2+����Ba2+����SO42-����������˵����Һ�в���SO42-��
��������ʵ����ʵȷ��������Һ�п϶����ڵ������ǣ�Ba2+��I-��NH4+���϶������ڵ������ǣ�Mg2+��Fe3+��Al3+��NO3-��Fe3+��CO32-��SO42-��������ȷ���Ƿ���ڵ������ǣ�K+��Cl-��
(1)����������Ӧ�����ӷ���ʽΪ��Cl2+2I-�TI2+2Cl-��
(2)������ʵ����ʵȷ��������Һ�п϶����ڵ������ǣ�Ba2+��I-��NH4+��
(3)����Һ�п϶������ڵ�������Mg2����Al3����Fe3����SO42-��CO32-��NO3-��
(4)������ȷ���Ƿ���ڵ������ǣ�K+��Cl-��

����Ŀ���й������ĸ����õ绯ѧװ�õ������У���ȷ����
|
|
|
|
ͼ�����п�̵�� | ͼ��Ǧ-�������� | ͼ���⾫��ͭ | ͼ����пŦ�۵�� |
A. ͼ����ʾ����У�MnO2�������Ǵ���
B. ͼ����ʾ��طŵ�����У�����Ũ�Ȳ�������
C. ͼ����ʾװ�ù��������У��������Һ��Cu2+Ũ��ʼ�ղ���
D. ͼ����ʾ����У�Ag2O������������ع��������л�ԭΪAg