��Ŀ����
�����±�������������ȷ���ǣ�
| ��� | ������ | ��ԭ�� | ������Ӧ�� | �������� | ��ԭ���� |
| �� | Cl2 | FeBr2 | FeCl3 | ||
| �� | KMnO4 | H2O2 | H2SO4 | O2 | MnSO4 |
| �� | KClO3 | HCl��Ũ�� | Cl2 | Cl2 | |
| �� | KMnO4 | HCl��Ũ�� | Cl2 | MnCl2 |
A�����еڢ��鷴Ӧ����������һ��ֻ��FeCl3��ʵΪFe3+��
B�������ԱȽ�: KMnO4��Cl2��Fe3+��Br2��Fe2+
C����ԭ�ԱȽ�: H2O2��Mn2+��Cl-
D���ܵ����ӷ���ʽ��ƽ��H+�Ļ�ѧ������Ϊ16
D

��ѧ��һֱ�����ڡ��˹��̵������·����о���
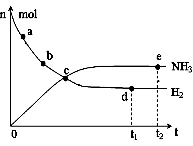 ��1��Ŀǰ�ϳɰ�����ԭ��Ϊ��N2(g) + 3H2(g)
��1��Ŀǰ�ϳɰ�����ԭ��Ϊ��N2(g) + 3H2(g)![]() 2NH3(g)��
2NH3(g)��
��H=��92.4kJ��mol��1��
�� 673K��30MPa�£������ϳɰ���Ӧ��n(NH3)��n(H2)��
ʱ��仯�Ĺ�ϵ����ͼ��ʾ������������ȷ���� ��
A����a������Ӧ���ʱȵ�b�Ĵ�
B����c����Ӧ�ﵽƽ��
C����d�͵� e����n(N2)��ͬ
D��773K��30MPa�£���Ӧ��t2ʱ�̴ﵽƽ�⣬��n(NH3)��ͼ��e���ֵ��
�� ���ݻ�Ϊ2.0 L���ݵ��ܱ������г���0.80 mol N2(g)��1.60 mol H2(g)����Ӧ��673K��30MPa�´ﵽƽ��ʱ��NH3���������Ϊ20%���������·�ӦN2(g) + 3H2(g)![]() 2NH3(g)��ƽ�ⳣ��K= ��KֵԽ������Ӧ�ﵽƽ��ʱ �������ţ���
2NH3(g)��ƽ�ⳣ��K= ��KֵԽ������Ӧ�ﵽƽ��ʱ �������ţ���
A����ѧ��Ӧ����Խ�� B��NH3�IJ���һ��Խ�� C������Ӧ���е�Խ��ȫ
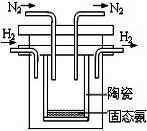 ��2��1998��ϣ������˹��´�ѧ����λ��ѧ�Ҳ��ø����ӵ�����
��2��1998��ϣ������˹��´�ѧ����λ��ѧ�Ҳ��ø����ӵ�����
�� SCY�մɣ��ܴ���H+����ʵ���˸��³�ѹ�¸�ת���ʵĵ�
��ϳɰ�����ʵ��װ����ͼ�������ĵ缫��Ӧʽ ��
��3���������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�
N2�ڴ�������������Fe2O3��TiO2��������ˮ�������з�Ӧ��
N2(g)+ 3H2O(1) ![]() 2NH3(g)+
2NH3(g)+ ![]() O2(g) ��H = a kJ��mol��1
O2(g) ��H = a kJ��mol��1
��һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±���
| T/K | 303 | 313 | 323 |
| NH3������/��10-6mol�� | 4.8 | 5.9 | 6.0 |
�ٴ˺ϳɷ�Ӧ��a 0����S 0������������������������÷�Ӧ����
A��һ���Է� B��һ�����Է� C�������Է� D�������Է�
����֪��N2(g)+ 3H2(g)![]() 2NH3(g) ��H= ��92 .4kJ��mol��1
2NH3(g) ��H= ��92 .4kJ��mol��1
2H2(g) + O2(g) = 2H2O(l) = ��571.6kJ��mol��1
��N2(g)+ 3H2O(1) = 2NH3(g) + ![]() O2(g)��H= kJ��mol��1��
O2(g)��H= kJ��mol��1��
��ѧ��һֱ�����ڡ��˹��̵������·����о���
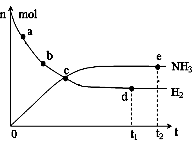 ��1��Ŀǰ�ϳɰ�����ԭ��Ϊ��N2(g) + 3H2(g)
��1��Ŀǰ�ϳɰ�����ԭ��Ϊ��N2(g) + 3H2(g) 2NH3(g)��
2NH3(g)��
��H=��92.4kJ��mol��1��
�� 673K��30MPa�£������ϳɰ���Ӧ��n(NH3)��n(H2)��
ʱ��仯�Ĺ�ϵ����ͼ��ʾ������������ȷ���� ��
A����a������Ӧ���ʱȵ�b�Ĵ�
B����c����Ӧ�ﵽƽ��
C����d�͵� e����n(N2)��ͬ
D��773K��30MPa�£���Ӧ��t2ʱ�̴ﵽƽ�⣬��n(NH3)��ͼ��e���ֵ��
�� ���ݻ�Ϊ2.0 L���ݵ��ܱ������г���0.80 mol N2(g)��1.60 mol H2(g)����Ӧ��673K��30MPa�´ﵽƽ��ʱ��NH3���������Ϊ20%���������·�ӦN2(g) + 3H2(g)
 2NH3(g)��ƽ�ⳣ��K= ��KֵԽ������Ӧ�ﵽƽ��ʱ �������ţ���
2NH3(g)��ƽ�ⳣ��K= ��KֵԽ������Ӧ�ﵽƽ��ʱ �������ţ���
A����ѧ��Ӧ����Խ�� B��NH3�IJ���һ��Խ�� C������Ӧ���е�Խ��ȫ
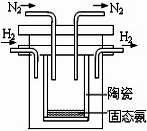 ��2��1998��ϣ������˹��´�ѧ����λ��ѧ�Ҳ��ø����ӵ�����
��2��1998��ϣ������˹��´�ѧ����λ��ѧ�Ҳ��ø����ӵ�����
�� SCY�մɣ��ܴ���H+����ʵ���˸��³�ѹ�¸�ת���ʵĵ�
��ϳɰ�����ʵ��װ����ͼ�������ĵ缫��Ӧʽ ��
��3���������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�
N2�ڴ�������������Fe2O3��TiO2��������ˮ�������з�Ӧ��
N2(g)
+ 3H2O(1)  2NH3(g)
+
2NH3(g)
+  O2(g)
��H = a kJ��mol��1
O2(g)
��H = a kJ��mol��1
��һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±���
|
T/K |
303 |
313 |
323 |
|
NH3������/��10-6mol�� |
4.8 |
5.9 |
6.0 |
�ٴ˺ϳɷ�Ӧ��a 0����S 0������������������������÷�Ӧ����
A��һ���Է� B��һ�����Է� C�������Է� D�������Է�
����֪��N2(g)
+ 3H2(g) 2NH3(g)
��H= ��92 .4kJ��mol��1
2NH3(g)
��H= ��92 .4kJ��mol��1
2H2(g) + O2(g) = 2H2O(l) = ��571.6kJ��mol��1
��N2(g)
+ 3H2O(1) = 2NH3(g) +  O2(g)
��H= kJ��mol��1��
O2(g)
��H= kJ��mol��1��
 2NH3(g)�� ��H=-92.4kJ��mol-1��
2NH3(g)�� ��H=-92.4kJ��mol-1�� 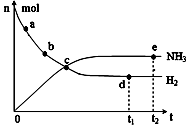
 2NH3(g)��ƽ�ⳣ��K= ________��������С��һλ��KֵԽ������Ӧ�ﵽƽ��ʱ_________�����ţ���
2NH3(g)��ƽ�ⳣ��K= ________��������С��һλ��KֵԽ������Ӧ�ﵽƽ��ʱ_________�����ţ���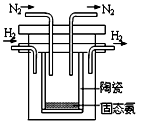
 2NH3(g) + O2(g)����H = a kJ��mol-1 ��һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±�
2NH3(g) + O2(g)����H = a kJ��mol-1 ��һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±� 
 2NH3(g) ��H= ��92 .4kJ��mol-1
2NH3(g) ��H= ��92 .4kJ��mol-1  O2(g) ��H=_____________kJ��mol-1
O2(g) ��H=_____________kJ��mol-1