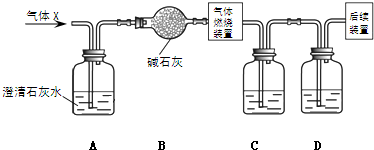
��Ŀ����
ij��ɫ����A�п��ܺ���H2S��SO2��CO2��HCl�����е�һ�ֻ���
(1)��������ͨ����ˮ����Һ�����ɫ����
(2)���õ�����Һ��Ϊ���ݣ�
������һ��ȡ���������Թ��м����������ữ��BaCl2��Һ�����ְ�ɫ������
����һ��ȡ�����������������ữ��AgNO3��Һ��Ҳ�õ���ɫ������
�Իش�
(1)����A�п϶����е�������________��������__________________________________��
(2)����A�п϶������ڵ�������________��������______________________________��
(3)����A�в���ȷ���Ƿ���ڵ�������________________________________________��
(1)SO2������ͨ����ˮ����Һ�����ɫ�����Ҽ����������ữ��BaCl2��Һ�����ְ�ɫ������
(2)H2S��H2S��SO2������
(3)CO2��HCl
����������1�����������ֻ��SO2�ɺ���ˮ��Ӧ�����ҿ�ʹ��ˮ��Һ�����ɫ����
Cl2��SO2��2H2O=2HCl��H2SO4
��2������H2S��SO2���ܹ��棺2H2S��SO2=3S��2H2O�����Ի�������п϶�������H2S
(3)ͨ���Ѹ�������ȷ��CO2��HCl������Ĵ��ڡ���Ϊ����ˮ�����Һ����������������ᣬ���Լ����������ữ��BaCl2��Һ��һ�������BaSO4��ɫ�����������������ữ��AgNO3��Һһ�������AgCl��ɫ������

���и���ij��ɫ��Һ��ʵ�������������Ľ�����һ������ȷ����
| A����ɫ��Ӧ�ʻ�ɫ�����ۣ���Һ��һ������Na+ |
| B�����������ữ���Ȼ�����Һ�������ְ�ɫ���������ۣ���Һ��һ������SO42�� |
| C����Ũ����������Һ����ʹ��ɫʯ����ֽ���������壬���ۣ�����Һһ����NH4+ |
| D������̼������Һ������ɫ���������ۣ���Һ�п��ܺ��������� |