��Ŀ����
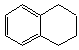
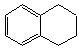
1��2��3��4-���⻯���Ľṹ��ʽ�� 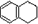 ������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2��C10H8Br4+4HBr�����ɵ����廯��������Ϊ��̬��������ˮ�����������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ��ʵ�鲽�����£�
������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2��C10H8Br4+4HBr�����ɵ����廯��������Ϊ��̬��������ˮ�����������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ��ʵ�鲽�����£�

�ٰ�һ�������Ȱ����⻯����ˮ�����ʵ��������У��������������ۣ�
����������Һ�壬���Ͻ��裬ֱ����Ӧ��ȫ��
��ȡ�·�Ӧ�����������������⻯����ֱ����Һ��ɫ��ʧ�����ˣ�����Һ�����Һ©�������ã�
�ܷ�Һ���õ��ġ�ˮ�㡱����������Һ���ش��������⣺
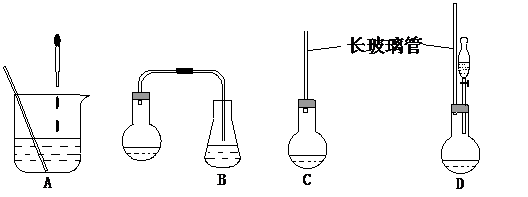
��1������ͼ��ʾ��װ�ã��ʺϲ���ٺ͢ڵIJ�������
��2��������в����������⻯����Ŀ����
��3��������й��˺�õ��Ĺ���������
��4���ڢܻ�õ�Ũ��Һ�м����Ҵ������ȣ��ɻ��һ�ֲ�����ˮ���л��д���÷�Ӧ�ķ���ʽ
��5����Ȳ�ܱ����Ը��������Һ����������ƽ����Ӧ�ķ���ʽ��
 ������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2��C10H8Br4+4HBr�����ɵ����廯��������Ϊ��̬��������ˮ�����������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ��ʵ�鲽�����£�
������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2��C10H8Br4+4HBr�����ɵ����廯��������Ϊ��̬��������ˮ�����������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ��ʵ�鲽�����£�
�ٰ�һ�������Ȱ����⻯����ˮ�����ʵ��������У��������������ۣ�
����������Һ�壬���Ͻ��裬ֱ����Ӧ��ȫ��
��ȡ�·�Ӧ�����������������⻯����ֱ����Һ��ɫ��ʧ�����ˣ�����Һ�����Һ©�������ã�
�ܷ�Һ���õ��ġ�ˮ�㡱����������Һ���ش��������⣺
��1������ͼ��ʾ��װ�ã��ʺϲ���ٺ͢ڵIJ�������
D
D
����2��������в����������⻯����Ŀ����
��Ӧ�������Br2
��Ӧ�������Br2
����3��������й��˺�õ��Ĺ���������
���廯��������
���廯��������
����4���ڢܻ�õ�Ũ��Һ�м����Ҵ������ȣ��ɻ��һ�ֲ�����ˮ���л��д���÷�Ӧ�ķ���ʽ
CH3CH2OH+HBr
CH3CH2Br+H2O
| �� |
CH3CH2OH+HBr
CH3CH2Br+H2O
��ʵ������ȡ��Ȳ������ͼ�е�װ��D����ʱ��Һ©���е�Һ��ͨ����| �� |
����ʳ��ˮ
����ʳ��ˮ
����5����Ȳ�ܱ����Ը��������Һ����������ƽ����Ӧ�ķ���ʽ��
1
1
C2H2+2
2
KMnO4+3
3
H2SO4=1
1
K2SO4+2
2
MnSO4+2
2
CO2+4
4
H2O����������1�����ݢ٢ڵIJ�����ʹ���Լ����ص��жϺ�����װ�ã�
��2�����ݲ������Ҫ�����˹�����Һ������жϣ�
��3���������廯��������Ϊ��̬�ͼ����������������ǣ�
��4���Ҵ����廯�ⷴӦ�����������飻��ȡ��Ȳͨ��ʹ��̼���ƺͱ���ʳ��ˮ��
��5�����ݷ�Ӧ����ʽ��Ԫ�صĻ��ϼ����������ƽ��Ӧ����ʽ��
��2�����ݲ������Ҫ�����˹�����Һ������жϣ�
��3���������廯��������Ϊ��̬�ͼ����������������ǣ�
��4���Ҵ����廯�ⷴӦ�����������飻��ȡ��Ȳͨ��ʹ��̼���ƺͱ���ʳ��ˮ��
��5�����ݷ�Ӧ����ʽ��Ԫ�صĻ��ϼ����������ƽ��Ӧ����ʽ��
����⣺��1�����ղ���٣���ʵ���ǰ����⻯����ˮ�����ۼ���ͬһ�����з�Ӧ������B����ȷ������A�е�װ�ý��з�Ӧ���������ʹ̼�����ζ�����⻯��������Ⱦ������ͬ��Cװ���ڲ���ڷ�Ӧʱ��Ҳ����ɻ�����Ⱦ������A��C�����ԣ���ѡDװ�ã�
�ʴ�Ϊ��D��
��2�����ڲ���ڼ����˹�����Һ�壬������в����������⻯������ʣ����嵥�ʣ�
�ʴ�Ϊ����Ӧ�������Br2��
��3���������ɵ����廯��������Ϊ��̬�����Թ��˺�õ��Ĺ������������廯�������ۣ�
�ʴ�Ϊ�����廯�������ۣ�
��4���ڢܻ�õ�Ũ��Һ�к����廯�⣬�廯�����Ҵ��ڼ������������������飬��Ӧ�ķ���ʽΪ��CH3CH2OH+HBr
CH3CH2Br+H2O��
ʵ������ȡ��Ȳ������ͼ�е�װ��D��Ϊ�˼�С��Ӧ���ʣ���ʱ��Һ©���е�Һ��ͨ��ʹ�ñ���ʳ��ˮ��
�ʴ�Ϊ��CH3CH2OH+HBr
CH3CH2Br+H2O������ʳ��ˮ��
��5����Ӧ�У����ϼ۱仯������Ȳ������أ���Ȳ�е�CԪ�ػ��ϼ۱仯Ϊ��-1��+4�����ϼ�����[4-��-1��]��2=10�ۣ���������е�MnԪ�ػ��ϼ۱仯Ϊ��+7��+2�����ϼ۽���5�ۣ����ϼ۱仯����С������Ϊ10��������Ȳ�Ļ�ѧ������Ϊ1��������صļ�����Ϊ2��Ȼ�����ù۲취��ƽ�������ʣ�
��ƽ��Ļ�ѧ����ʽΪ��C2H2+2KMnO4+3H2SO4=K2SO4+2MnSO4+2CO2+4H2O��
�ʴ�Ϊ��1��2��3��1��2��2��4��
�ʴ�Ϊ��D��
��2�����ڲ���ڼ����˹�����Һ�壬������в����������⻯������ʣ����嵥�ʣ�
�ʴ�Ϊ����Ӧ�������Br2��
��3���������ɵ����廯��������Ϊ��̬�����Թ��˺�õ��Ĺ������������廯�������ۣ�
�ʴ�Ϊ�����廯�������ۣ�
��4���ڢܻ�õ�Ũ��Һ�к����廯�⣬�廯�����Ҵ��ڼ������������������飬��Ӧ�ķ���ʽΪ��CH3CH2OH+HBr
| �� |
ʵ������ȡ��Ȳ������ͼ�е�װ��D��Ϊ�˼�С��Ӧ���ʣ���ʱ��Һ©���е�Һ��ͨ��ʹ�ñ���ʳ��ˮ��
�ʴ�Ϊ��CH3CH2OH+HBr
| �� |
��5����Ӧ�У����ϼ۱仯������Ȳ������أ���Ȳ�е�CԪ�ػ��ϼ۱仯Ϊ��-1��+4�����ϼ�����[4-��-1��]��2=10�ۣ���������е�MnԪ�ػ��ϼ۱仯Ϊ��+7��+2�����ϼ۽���5�ۣ����ϼ۱仯����С������Ϊ10��������Ȳ�Ļ�ѧ������Ϊ1��������صļ�����Ϊ2��Ȼ�����ù۲취��ƽ�������ʣ�
��ƽ��Ļ�ѧ����ʽΪ��C2H2+2KMnO4+3H2SO4=K2SO4+2MnSO4+2CO2+4H2O��
�ʴ�Ϊ��1��2��3��1��2��2��4��
���������⿼���������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ���漰��װ�õ�ѡ��ѧ����ʽ����д����ƽ����ֿ�����ѧ���ķ�������������������ѧ֪ʶ���յ������̶ȣ������Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 ������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2
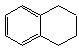
������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2 C10H8Br4+4HBr�����ɵ����廯��������Ϊ��̬��������ˮ�����������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ��ʵ�鲽�����£�
C10H8Br4+4HBr�����ɵ����廯��������Ϊ��̬��������ˮ�����������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ��ʵ�鲽�����£�

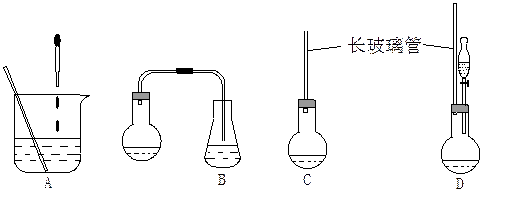
 ������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2
������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2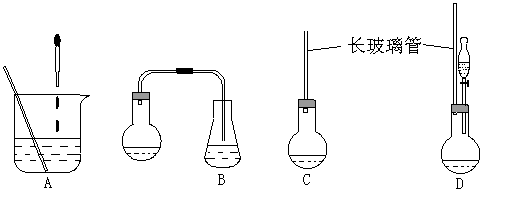
 ������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2
������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2