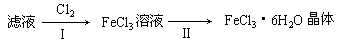��Ŀ����
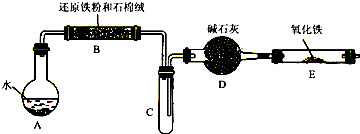
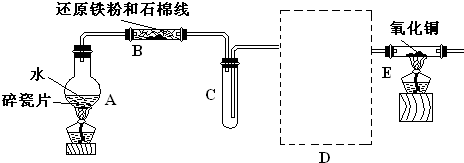
ѧ��������ͼ����װ�ý��С�����ˮ������Ӧ����ʵ�飬�����ò����һ����ȡFeCl3��6H2O���塣��ͼ�мгּ�β������װ�þ�����ȥ��
(1) �ش��������⣺�����������������ʣ�����ȥ���������л��е����ۿ���ѡ�õ��Լ�Ϊ__ __������ţ���
A��ϡ���� B������������Һ C��Ũ���� D. FeCl3��Һ
�����Ӻ�����۽�һ�������װ�뷴Ӧ������
��2����Ӧ������װ��B�з�����Ӧ�Ļ�ѧ����ʽ��_______________ ____��
Dװ�õ����ã�_______________________.
��3����С��ѧ����B�з�Ӧ��IJ���������������ᣬ���ˣ���������Һ��ȡFeCl3��6H2O���壬����������£�
![]()
�ٲ���I��ͨ��Cl2������
�������������ԭ�������������ԭ���
��Ϊ�˼���ijδ֪��Һ�Ƿ���FeCl2��Һ��ͬѧ�����������ʵ�鷽������֤����
��һ֧װ�и�δ֪��Һ���Թ�����ͨ���������ٵμ�KSCN��Һ����Һ���ֺ�ɫ��֤����δ֪��Һ��FeCl2��Һ������Ϊ�˷����Ƿ���� (���������������)��
(1) B
(2) 3Fe + 4H2O Fe3O4+ 4H2 . ��ȥH2�е�ˮ����
��3���� ������ �ڡ�������
����:

 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�