��Ŀ����
������Һ���й����ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
A��NaHSO3��Һ�����ԣ����У�c��Na������c��HSO3-����c��SO32-����c��H������c��OH����
B��pH��ȵ�CH3COONa��Na2CO3������Һ��c��CH3COONa����c��Na2CO3��
C��ǿ��HA��Һ������MOH��Һ��Ϻ���Һ�����ԣ����У�c��M������c��A����
D��0.1 mol��L��1��NaHA��ҺpH��1�����У�c��Na������c��H2A����c��HA������c��A2����
C
����

���и���Һ�У����ӵ����ʵ���Ũ�ȹ�ϵ��ȷ����
| A��(NH4)2SO4��Һ�� c(SO42-)>c(NH4+)>c(H+)>c(OH-) |
| B��Na2Sϡ��Һ�� c(Na+)��c(S2-)+c(H2S)+c(HS-) |
| C������ʳ��ˮ�� c(Na+)+c(H+)��c(Cl-)+c(OH-) |
| D��CH3COOH��Һ��ˮϡ�ͺ���Һ����������Ũ�ȶ���С |
�����йص������Һ������������ȷ����
| A��ϡ�����ˮϡ�ͣ��������̶�������Һ��pH���� |
| B����CH3COONa��Һ�м�������CH3COOH����ʹc��Na+��=c��CH3 COO���� |
| C���ں���BaSO4��������Һ�м���Na2SO4���壬��Һ��c��Ba2+����С |
| D�������£�pH =2��������pH =12�İ�ˮ��������������Һ�У�c��Cl����>c��NH4+��>c��H+��>c��OH���� |
��֪0.1 mol/L�Ĵ�����Һ�д��ڵ���ƽ��:CH3COOH CH3COO-+H+����ʹƽ�ⷢ����ͼ�仯,���Բ�ȡ�Ĵ�ʩ��(�� ��)
CH3COO-+H+����ʹƽ�ⷢ����ͼ�仯,���Բ�ȡ�Ĵ�ʩ��(�� ��)
| A���������ռ���Һ | B�������¶� |
| C�������������� | D����ˮ |
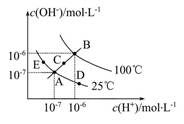
������Һ���й��������ʵ���Ũ�Ȼ���������ϵ��ȷ����(�� ��)
A��NaHSO3��NaHCO3�����Ի����Һ��(S��C����R��ʾ):c(Na+)=c(HR )+2c(R )+2c(R ) ) |
| B�������½������ơ���������Һ��Ϻ�,��Һ������,���Ϻ���Һ��:c(Na+)��c(Cl-)��c(CH3COOH) |
C�����������ʵ���Ũ����ȵĢ�(NH4)2CO3����(NH4)2SO4����(NH4)2Fe(SO4)2������Һ��c(N ):�٣��ۣ��� ):�٣��ۣ��� |
| D������������ʵ���Ũ�ȵ�NaClO��Һ��NaCl��Һ����������:Nǰ��N�� |
ij������Һ��ֻ����Na����CH3COO����H����OH�� 4�����ӡ�����������ȷ���ǣ���������
| A������Һ�����ɵ����ʵ���Ũ�ȡ��������NaOH��Һ��CH3COOH��Һ��϶��� |
| B������Һһ����pH��3��CH3COOH��Һ��pH��11��NaOH��Һ�������϶��� |
| C������Һ������Ũ��һ��Ϊc��Na����>c��CH3COO����>c��OH����>c��H���� |
| D������һ���������ᣬc��CH3COO�������ܴ��ڡ����ڻ�С��c��Na���� |
���и���Һ�У��������ʵ���Ũ�ȹ�ϵ������ȷ����(����)
| A��0.1 mol��L��1 Na2CO3��Һ�У�c(Na��)��c(HCO3��)��c(H2CO3)��2c(CO32��) |
| B�������£�pH��4�Ĵ�����pH��10��NaOH��Һ�������Ϻ�pH<7 |
| C����0.2 mol��L��1 NaA��Һ��0.1 mol��L��1������Һ�����������ü�����Һ�У�c(Na��)��c(H��)��c(A��)��c(Cl��) |
| D��pH��12��Ba(OH)2��Һ��pH��12��Na2CO3��Һ�У�ˮ�����c(H��)��� |
25 ��ʱ��x mol��L��1�����ˮ��Һ�У�����KOH���������Һ��pH(���Լ�������������Һ����仯)���õ�c(HF)��c(F��)����ҺpH�ı仯��ϵ��ͼ��ʾ������˵����ȷ���� (����)��
| A����c(F��)>c(HF)ʱ����Һ�ʼ��� |
| B����pH��2ʱ��c(F��)<c(K��) |
| C��25 ��ʱ�������ĵ���ƽ�ⳣ��Ϊ4��10��6 |
| D����pH��5ʱ��c(HF)��c(K��)��c(H��)��c(OH��)��x mol��L��1 |