��Ŀ����
��450�沢�д��������£������Ϊ1L���ܱպ��������У���������������������·�Ӧ��2SO2+O2 2SO3
2SO3
��1����֪��64g SO2��ȫת��ΪSO3��ų�85kJ������SO2ת��ΪSO3���Ȼ�ѧ����ʽ�� ��
��2���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK= ��
��3�������¶ȣ���ѧ��Ӧ�ٶ� ���÷�ӦKֵ�� ��ѹǿ�� ���������С�����䡱��
��4��450��ʱ����һ�ܱ������У�������������������ϣ���Ӧ������SO2��O2��SO3���ʵ����仯��ͼ����Ӧ����ƽ��״̬��ʱ����� ��
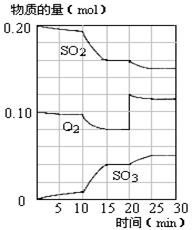
a��10-15min b��15-20min c��20-25min d��25-30min
��5����ͼ�жϣ�10min��15min�����߱仯��ԭ������� ����д��ţ���
a������SO3�����ʵ��� b����С������� c�������¶� d.����
��6����15����ʱ��SO2��ת������ ��
 2SO3
2SO3��1����֪��64g SO2��ȫת��ΪSO3��ų�85kJ������SO2ת��ΪSO3���Ȼ�ѧ����ʽ�� ��
��2���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK= ��
��3�������¶ȣ���ѧ��Ӧ�ٶ� ���÷�ӦKֵ�� ��ѹǿ�� ���������С�����䡱��
��4��450��ʱ����һ�ܱ������У�������������������ϣ���Ӧ������SO2��O2��SO3���ʵ����仯��ͼ����Ӧ����ƽ��״̬��ʱ����� ��

a��10-15min b��15-20min c��20-25min d��25-30min
��5����ͼ�жϣ�10min��15min�����߱仯��ԭ������� ����д��ţ���
a������SO3�����ʵ��� b����С������� c�������¶� d.����
��6����15����ʱ��SO2��ת������ ��
��1��SO2(g)+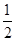 O2(g) = SO3(g)����H = -85kJ/mol����2��
O2(g) = SO3(g)����H = -85kJ/mol����2��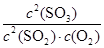
��3������������4��b d��5��c��6��20%
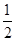 O2(g) = SO3(g)����H = -85kJ/mol����2��
O2(g) = SO3(g)����H = -85kJ/mol����2��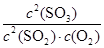
��3������������4��b d��5��c��6��20%
�����������1��������֪��64g SO2Ϊ1mol����ȫת��ΪSO3��ų�����85kJ�������Ȼ�ѧ��Ӧ����ʽSO2(g)+
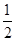 O2(g)=SO3(g)����H = -85kJ/mol����2�����ݻ�ѧƽ�ⳣ������ʽ����K=
O2(g)=SO3(g)����H = -85kJ/mol����2�����ݻ�ѧƽ�ⳣ������ʽ����K=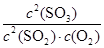 ��3���������Ȼ�ѧ��Ӧ����ʽ������ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ�����ѹǿ��С��ƽ�ⳣ��k�������¶ȷ�Ӧ���ʼ�С��4����ƽ��״̬�����������ʵ���Ũ�Ȳ��ٱ仯����ͼ���б���Ϊ���ߣ���bd��ȷ��5����10min��15min�����߱仯��֪��Ӧ�����ʵ����������������ʵ������ߣ���Ӧ������У�����ab�·�Ӧ������У�����c�·�Ӧ������У�����d�²��ı����ʵ����仯����c��ȷ��6��������֪����15����ʱ����Ӧ��SO2���ʵ���Ϊ0.04mol����ʼ���ʵ���Ϊ0.2mol����SO2��ת����Ϊ20%��
��3���������Ȼ�ѧ��Ӧ����ʽ������ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ�����ѹǿ��С��ƽ�ⳣ��k�������¶ȷ�Ӧ���ʼ�С��4����ƽ��״̬�����������ʵ���Ũ�Ȳ��ٱ仯����ͼ���б���Ϊ���ߣ���bd��ȷ��5����10min��15min�����߱仯��֪��Ӧ�����ʵ����������������ʵ������ߣ���Ӧ������У�����ab�·�Ӧ������У�����c�·�Ӧ������У�����d�²��ı����ʵ����仯����c��ȷ��6��������֪����15����ʱ����Ӧ��SO2���ʵ���Ϊ0.04mol����ʼ���ʵ���Ϊ0.2mol����SO2��ת����Ϊ20%��
��ϰ��ϵ�д�
 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
�����Ŀ
 N2(g)��2CO2(g)��H����746.8 kJ��mol��1���ں��ݵ��ܱ������У���Ӧ�ﵽƽ����ı�����һ������X��Y��X�ı仯������ͼ�����ߵ���( )
N2(g)��2CO2(g)��H����746.8 kJ��mol��1���ں��ݵ��ܱ������У���Ӧ�ﵽƽ����ı�����һ������X��Y��X�ı仯������ͼ�����ߵ���( )
 2SO3��H��0���Ʊ����ᣬ���жԸ÷�Ӧ��˵����ȷ����
2SO3��H��0���Ʊ����ᣬ���жԸ÷�Ӧ��˵����ȷ���� TaI4��g��+S2��g�� ����
TaI4��g��+S2��g�� ����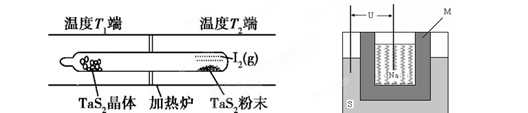
 2C��g�� ��H��0�������������仯��ֻ���¶ȱ仯ʱ��ij�����¶ȱ仯�Ĺ�ϵ��ͼ��ʾ��������˵���У���ȷ����
2C��g�� ��H��0�������������仯��ֻ���¶ȱ仯ʱ��ij�����¶ȱ仯�Ĺ�ϵ��ͼ��ʾ��������˵���У���ȷ����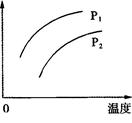
 FeO(s)+CO(g)
FeO(s)+CO(g) xC(g) ��H �� 0��B��C�����ʵ�����ʱ��仯�Ĺ�ϵ��ͼ��ʾ�������й�˵������ȷ����
xC(g) ��H �� 0��B��C�����ʵ�����ʱ��仯�Ĺ�ϵ��ͼ��ʾ�������й�˵������ȷ����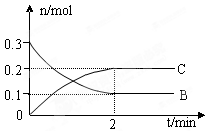
 2C(g)�ﵽƽ���־����
2C(g)�ﵽƽ���־����