��Ŀ����
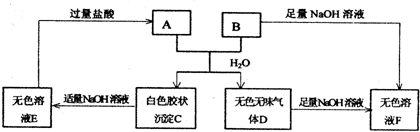
��8�֣��ɶ�����Ԫ����ɵ�A��B��C��D��E��F������������ֻ��C��D�Ƿ��ӣ��������־�Ϊ��һ����λ����ɵ������ӻ�һ����λ����ɵ������ӣ���ÿ�����ж�����10�����ӡ���֪A��E���ɷǽ���Ԫ����ɵ������ӣ��������������й�ϵ��
��A����B�� C��D�� B����E����2
C��D�� B����E����2 D��
D��
��ͨ���� ���£�C��һ����ɫ�̼�����ζ�����壬�ҿ�ʹʪ��ĺ�ɫʯ
���£�C��һ����ɫ�̼�����ζ�����壬�ҿ�ʹʪ��ĺ�ɫʯ ����ֽ������
����ֽ������
����F���ӵ���Һ�м���C��ˮ��Һ�������ɰ�ɫ����W��C��Һ��������Ҳ����ʧ���ټ��뺬����B���ӻ����E���ӵ���Һʱ������W������һ��������ܽ⣬��һ������²��ܽ⡣
��ش��������⣺
��1����A�����Ƽ���ѧʽ�ֱ�Ϊ �� ��
��2��C��ˮ��Һ�д��ڵķ������������� �֡�
��3��д����F�����C��ˮ��Һ��Ӧ�����ӷ���ʽ�� ��
��4������0.1mol F���ӵ�50mL��Һ�У����뺬1.5mol/L B���ӵ�200mLǿ����Һ������а�ɫ��������������û�����м��뺬1mol/L E���ӵ�ǿ����Һ����Ҫʹ����ǡ���ܽ⣬�����������Һ���������Ϊ mL��
��8�֣�
��1��泥��������ӡ�NH4�� ��ÿ��1�֣���2�֣�
��2��3 ��2�֣�
��3��Mg2����2NH3��H2O��Mg(OH)2����2NH4�� ��2�֣�
��4��300 ��2�֣�
����


 A��B�����οɷ������б仯����B����ɫ��Ӧ�ʻ�ɫ��ͼ������������δ�г���
A��B�����οɷ������б仯����B����ɫ��Ӧ�ʻ�ɫ��ͼ������������δ�г���