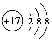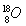��Ŀ����
ʵ�������ú�����CO2���ʵ�CO����ԭ����������FexOy��֤��CO�ܹ���ԭFexOy���ұ���������ΪCO2��ʵ�����ṩ�ĸ���������ҩƷ���£�
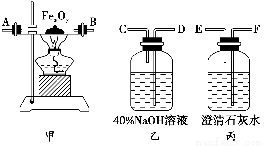
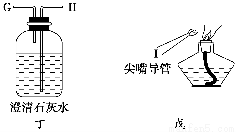
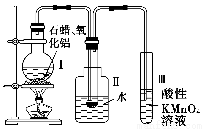
(1)ʵ��ʱ����������װ�õ���ȷ����˳����(��д���ӿڵĴ���)����������D��________��________��(F)��(E)��________��________��________��________��________��
(2)��װ�����з�����Ӧ�Ļ�ѧ����ʽ��_______________________��
(3)��װ�ü��з�����Ӧ�Ļ�ѧ����ʽ��______________��
(4)��װ���г���ʯ��ˮ��������_______________��
(5)�������ⶨ��0.4 g FexOy��CO��Ӧ���ɵ�����ͨ������ij���ʯ��ˮ�У�����0.75 g��ɫ��������FexOy��xֵ��________��yֵ��________��
(6)ʵ������У���˵��CO�ܹ���ԭFexOy��ʵ��������___________���ܹ�˵��CO��������CO2��ʵ��������____________��
(1)D��C��A��B��H��G��I
(2)2NaOH��CO2=Na2CO3��H2O
(3)FexOy��yCO xFe��yCO2
xFe��yCO2
(4)����CO2�Ƿ����
(5)2��3
(6)�����ĩ�Ӻ�ɫ���ɫ�����г���ʯ��ˮ�����
����������ʵ��Ŀ�ģ�����ÿ��װ�õ����ã�Ȼ�����ӣ�x��yֵ�ļ�������C�غ��ϵCO��CO2��CaCO3
n(CaCO3)��n(CO)��0.75/100��7.5��10��3mol
FexOy����������Ϊ44 g��mol��1��7.5��10��3mol��28 g��mol��1��7.5��10��3mol��0.12 g
n(Fe)��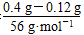 ��0.005 mol��
��0.005 mol��
n(O)��0.12/16��0.007 5 mol��
x/y��2/3

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�