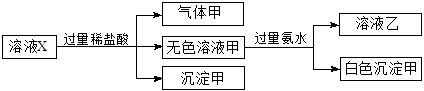
��Ŀ����
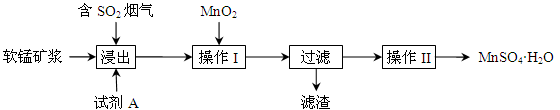
��16�֣������Ѱ�ҵ�ĸ���ƷFeSO4����TiO2+��Al3+������������ؼ��ߴ���ϸ�����������乤���������£�
��1������FeSO4�Ƿ����в��������ķ����� ��
��2����֪����1�õ�����������Ҫ�ɷ���Al(OH)3��H2TiO3���벹�仯ѧ����ʽ��
TiOSO4 + ��H2SO4 + H2TiO3�������۵������У��ٳ�ȥ��Һ�е�Fe3+���� ��
��3��������Ӧ�����ӷ���ʽ�� ��
��4���������̵ķ�Ӧ�¶�Ϊ40�棬�¶Ȳ��˹��ߵ�ԭ����˿��Ƴ����������⣬���� ��FeC2O4���ɺ�Ϊ��߲�Ʒ���ȣ����������ҺpH��2����pH���ͣ�����FeC2O4�IJ���______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��5������2�õ�����Һ������Ũ���� ��ϴ�ӿɵõ�����Ʒ�������ʿ����� (д��һ����;)��

��1������FeSO4�Ƿ����в��������ķ����� ��
��2����֪����1�õ�����������Ҫ�ɷ���Al(OH)3��H2TiO3���벹�仯ѧ����ʽ��
TiOSO4 + ��H2SO4 + H2TiO3�������۵������У��ٳ�ȥ��Һ�е�Fe3+���� ��
��3��������Ӧ�����ӷ���ʽ�� ��
��4���������̵ķ�Ӧ�¶�Ϊ40�棬�¶Ȳ��˹��ߵ�ԭ����˿��Ƴ����������⣬���� ��FeC2O4���ɺ�Ϊ��߲�Ʒ���ȣ����������ҺpH��2����pH���ͣ�����FeC2O4�IJ���______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��5������2�õ�����Һ������Ũ���� ��ϴ�ӿɵõ�����Ʒ�������ʿ����� (д��һ����;)��
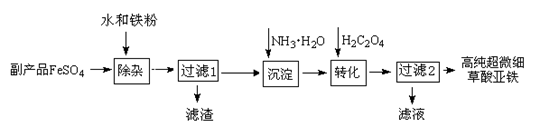
(16�֣�ÿ��2�֣���1��ȡ��������Ʒ���Թ��У�����������ˮ�ܽ⣬�μ�1��2��KSCN��Һ������Һ�Ժ�ɫ���������в���������������ȫ��1�֣�
��2��2H2O����д��H2O ��1�֣� ����Һ�е�H+��Ӧ��ʹAl3+ˮ����ȫ����Al(OH)3����
��3��2NH3?H2O + Fe2+ ��Fe(OH)2��+2NH4+����ƽ�����©�������ſ�1�֣�
��4��NH3?H2O��Fe(OH)2���ȶ��ֽ⣻ƫ�� ��5����ȴ�ᾧ�����ˣ�����
��2��2H2O����д��H2O ��1�֣� ����Һ�е�H+��Ӧ��ʹAl3+ˮ����ȫ����Al(OH)3����
��3��2NH3?H2O + Fe2+ ��Fe(OH)2��+2NH4+����ƽ�����©�������ſ�1�֣�
��4��NH3?H2O��Fe(OH)2���ȶ��ֽ⣻ƫ�� ��5����ȴ�ᾧ�����ˣ�����
�����������1���������ӱ��������������ӣ�����ͨ��������������֤�Ƿ����������Լ���FeSO4�Ƿ����в��������ķ�����ȡ��������Ʒ���Թ��У�����������ˮ�ܽ⣬�μ�1��2��KSCN��Һ������Һ�Ժ�ɫ���������в���������
��2�����ݷ�Ӧǰ��ԭ���غ��֪����ȱ��2����ˮ��������Һ�����ԣ��������к����������������Ҫ�õ������������뽵����Һ�����ԣ�����������һ����������Һ�е�H+��Ӧ��ʹAl3+ˮ����ȫ����Al(OH)3������
��3����Һ�е����������백ˮ��������������������������Ӧ�����ӷ���ʽΪ2NH3?H2O + Fe2+ ��Fe(OH)2��+2NH4+��
��4�����¶ȹ�����NH3?H2O��Fe(OH)2���ȶ��ֽ⣻��pH���ͣ�����FeC2O4�����ܽ⣬�Ӷ����²���ƫ�ͣ�
��5������2�õ�����Һ������Ũ������ȴ�ᾧ��ϴ�ӿɵõ�����Ʒ����泥������ʿ��������ʡ�

��ϰ��ϵ�д�
�����Ŀ
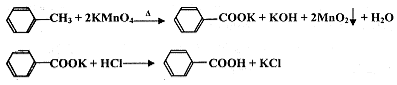
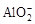 ��
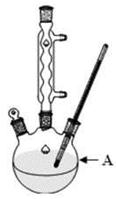
��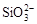 ��
��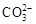 ��
��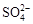 �е����������ӡ�ijͬѧ�Ը����н���������ʵ�飺
�е����������ӡ�ijͬѧ�Ը����н���������ʵ�飺