��Ŀ����
��2008�����������ۣ�28����ѧʵ������ȡ�Ȼ�������ķ���֮һ�ǽ�Ũ�������Ũ�����С������ͼ����ѡ�����������ڷ����ڻ����ø÷����Ʊ����ռ������Ȼ��������װ�ü�ͼ������ͼ�б��������Լ������������ظ�ʹ�ã��̶�װ�ò��ػ�������
| |
��1��д����ʵ���Ʊ����������Ļ�ѧ����ʽ���ߣ� �ߣߡ�
��2��������Һ��ϡ�ᵼ�£ߣ� �ߣߡ�
��3������ˮԡ���ȵ�ԭ���ǣߣ� �ߣߡ�
��4����Ӧʱ��м������Ŀ���ǣ������ӷ���ʽ��ʾ���ߣ� �ߣߡ�
��5����Һ���ȹ��˵�ԭ���ǣߣ� �ߡ������Թܿڵ�Ŀ���ǣߣߣ� �ߡ�
��6��������ȴһ��ʱ������Թ��й۲쵽�������ǣ� �ߣߣߡ�
��1��Fe+H2SO4��ϡ��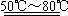 ��FeSO4+H2����װ��ͼ����������2����Ӧ�������������ھ�����������3����4��Fe3++2Fe��3Fe2+����5������FeSO4����ʧ����ֹ���������Թܽ�Fe2+����ΪFe3+����6����dz��ɫ����������
��FeSO4+H2����װ��ͼ����������2����Ӧ�������������ھ�����������3����4��Fe3++2Fe��3Fe2+����5������FeSO4����ʧ����ֹ���������Թܽ�Fe2+����ΪFe3+����6����dz��ɫ����������
����

��2008�����������ۣ�28����ѧʵ������ȡ�Ȼ�������ķ���֮һ�ǽ�Ũ�������Ũ�����С������ͼ����ѡ�����������ڷ����ڻ����ø÷����Ʊ����ռ������Ȼ��������װ�ü�ͼ������ͼ�б��������Լ������������ظ�ʹ�ã��̶�װ�ò��ػ�������

��ʵ�����Ʊ������������������ʵ�鲽�����£�ȡ�����ྻ����м������20%��30%��ϡ������Һ����50�棭80��ˮԡ�м��������ٲ������ݡ�����Һ���ȹ��ˣ���Һ�����Թ��У������������Թܿڣ����á���ȴһ��ʱ����ռ���Ʒ��
��1��д����ʵ���Ʊ����������Ļ�ѧ����ʽ���ߣ� �ߣߡ�
��2��������Һ��ϡ�ᵼ�£ߣ� �ߣߡ�
��3������ˮԡ���ȵ�ԭ���ǣߣ� �ߣߡ�
��4����Ӧʱ��м������Ŀ���ǣ������ӷ���ʽ��ʾ���ߣ� �ߣߡ�
��5����Һ���ȹ��˵�ԭ���ǣߣ� �ߡ������Թܿڵ�Ŀ���ǣߣߣ� �ߡ�
��6��������ȴһ��ʱ������Թ��й۲쵽�������ǣ� �ߣߣߡ�