��Ŀ����
��0.1 mol��L��1��NH4HSO4��Һ�У������������ʵ���Ũ�ȹ�ϵ����ȷ���� (����)
| A��c(H��)��c(SO42-)��c(NH4+) |
| B��c(NH4+)��c(H��)��2c(SO42-)��c(OH��) |
| C��c(H��)��c(NH4+)��c(NH3��H2O)��c(OH��) |
| D�������µμ�NaOH��Һ�����Ժ�c(Na��)��c(SO42-)��c(NH4+)��c(OH��)��c(H��) |
C
A�c(H��)��c(SO42-)��c(NH4+)����ȷ��B����ϵ���غ㣻C�NH4HSO4=NH4+��H����SO42-��NH4+��H2O NH3��H2O��H����H2O
NH3��H2O��H����H2O H����OH��������c(H��)��c(NH4+)��2c(NH3��H2O)��c(OH��)������D���NaOH��NH4HSO4��Ũ�ȵ������Ϻ�ʱ��Һ�����ԣ����Լ����NaOHӦ����NH4HSO4�����Ը�ѡ����ȷ��
H����OH��������c(H��)��c(NH4+)��2c(NH3��H2O)��c(OH��)������D���NaOH��NH4HSO4��Ũ�ȵ������Ϻ�ʱ��Һ�����ԣ����Լ����NaOHӦ����NH4HSO4�����Ը�ѡ����ȷ��
 NH3��H2O��H����H2O
NH3��H2O��H����H2O H����OH��������c(H��)��c(NH4+)��2c(NH3��H2O)��c(OH��)������D���NaOH��NH4HSO4��Ũ�ȵ������Ϻ�ʱ��Һ�����ԣ����Լ����NaOHӦ����NH4HSO4�����Ը�ѡ����ȷ��
H����OH��������c(H��)��c(NH4+)��2c(NH3��H2O)��c(OH��)������D���NaOH��NH4HSO4��Ũ�ȵ������Ϻ�ʱ��Һ�����ԣ����Լ����NaOHӦ����NH4HSO4�����Ը�ѡ����ȷ��
��ϰ��ϵ�д�
�����Ŀ
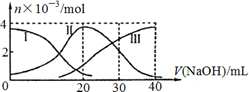
 H+ + HA����HA��
H+ + HA����HA��  H+ + A2��
H+ + A2�� ���ɼ����������(����)
���ɼ����������(����)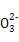 ),��c(Mg2+)��c(C
),��c(Mg2+)��c(C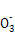 ��Ӳˮ,���������ӷ�Ӧ����ʽΪMg2++2HC
��Ӳˮ,���������ӷ�Ӧ����ʽΪMg2++2HC CaCO3��+MgCO3��+2H2O
CaCO3��+MgCO3��+2H2O