��Ŀ����
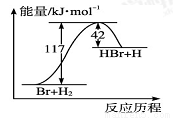
��ҵ���Ʊ����跴Ӧ���Ȼ�ѧ����ʽ���£�SiCl4��g����2H2��g�� Si��s����4HCl��g�� ��H��Q kJ/mol��Q��0��ij�¶ȡ�ѹǿ�£���һ������Ӧ��ͨ���ܱ������������Ϸ�Ӧ������������ȷ���ǣ� ��
Si��s����4HCl��g�� ��H��Q kJ/mol��Q��0��ij�¶ȡ�ѹǿ�£���һ������Ӧ��ͨ���ܱ������������Ϸ�Ӧ������������ȷ���ǣ� ��
A����Ӧ�����У�������ѹǿ�����SiCl4��ת����
B������Ӧ��ʼʱSiCl4Ϊ1 mol, ���ƽ��ʱ����������ΪQ kJ
C����Ӧ��4 minʱ����HClŨ��Ϊ0.12 mol/L����H2�ķ�Ӧ����Ϊ0.03 mol/��L��min��
D����Ӧ��������0.025Q kJʱ�����ɵ�HClͨ��100 mL 1 mol/L��NaOH��Һǡ�÷�Ӧ
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


 CO��g��+3H2��g�� ��H=+206.2kJ��mol-1
CO��g��+3H2��g�� ��H=+206.2kJ��mol-1 CO��g��+3H2��g��,CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ
CO��g��+3H2��g��,CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ
 00��ʱ�ﵽƽ�������ʱ��Ϊ5min������H2��ʾ��ƽ����Ӧ����_________��
00��ʱ�ﵽƽ�������ʱ��Ϊ5min������H2��ʾ��ƽ����Ӧ����_________�� B.
B.  C.
C.  D.
D. 
 xC��g����2D��g����2 min��÷�Ӧ�ﵽƽ�⣬����0.8 mol D�������C��Ũ��Ϊ0.2 mol/L�������жϴ�����ǣ� ��
xC��g����2D��g����2 min��÷�Ӧ�ﵽƽ�⣬����0.8 mol D�������C��Ũ��Ϊ0.2 mol/L�������жϴ�����ǣ� ��