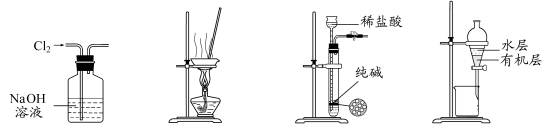
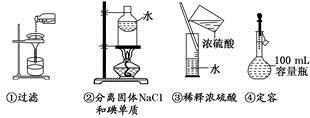
ĢāÄæÄŚČŻ
ĻĀĮŠŹµŃé²½ÖčÓėŹµŃéŹĀŹµµÄĻąÓ¦½įĀŪ½āŹĶÕżČ·µÄŹĒ
| Ń”Ļī | ŹµŃé²½ÖčÓėŹµŃéŹĀŹµ | ½įĀŪ½āŹĶ |
| A | Mg(OH)2”¢Al(OH)3»ģŗĻĪļÖŠ¼ÓČėNaOHČÜŅŗ£¬Al(OH)3Čܽā¶ųMg(OH)2ƻӊČܽā | Mg(OH)2Ksp±ČAl(OH)3µÄŠ” |
| B | ZnSÄÜČܽāŌŚĻ”ŃĪĖįÖŠ£¬CuS²»ÄÜČܽāŌŚĻ”ŃĪĖįÖŠ | CuSµÄKsp±ČZnSµÄŠ” |
| C | ŌŚČÜÓŠNH3µÄBaCl2ČÜŅŗÖŠĶØČėCO2£¬ÓŠ°×É«³ĮµķÉś³É | NH3ŌŚ·“Ó¦ÖŠ×÷“߻ƼĮ |
| D | µ„ÖŹ¹č²»ČÜÓŚÅØĮņĖįŗĶÅØĻõĖį | ¹č±»ĒæŃõ»ÆŠŌĖį¶Ū»Æ |
B
ŹŌĢā·ÖĪö£ŗA”¢Mg(OH)2”¢Al(OH)3»ģŗĻĪļÖŠ¼ÓČėNaOHČÜŅŗ£¬Al(OH)3Čܽā¶ųMg(OH)2ƻӊČܽāŹĒŅņĪŖAl(OH)3ĪŖĮ½ŠŌĒāŃõ»ÆĪļ£¬æÉÓėĒæ¼īNaOHČÜŅŗ·“Ó¦£¬“ķĪó£»B”¢ZnSÄÜČܽāŌŚĻ”ŃĪĖįÖŠ£¬CuS²»ÄÜČܽāŌŚĻ”ŃĪĖįÖŠŹĒÓÉÓŚCuSµÄKsp±ČZnSµÄŠ”£¬ÕżČ·£»C”¢ŌŚČÜÓŠNH3µÄBaCl2ČÜŅŗÖŠĶØČėCO2£¬ÓŠ°×É«³ĮµķÉś³ÉŹĒŅņĪŖ¶žŃõ»ÆĢ¼ÓėĖ®ŗĶNH3·“Ӧɜ³ÉµÄĢ¼Ėįļ§ÓėBaCl2ČÜŅŗ·“Ӧɜ³ÉĢ¼Ėį±µ³Įµķ£¬“ķĪó£»D”¢µ„ÖŹ¹č²»ČÜÓŚÅØĮņĖįŗĶÅØĻõĖįŹĒÓÉÓŚµ„ÖŹ¹čŠŌÖŹĪČ¶Ø£¬ÓėÅØĮņĖį”¢ÅØĻõĖį²»·“Ó¦£¬“ķĪó”£

Į·Ļ°²įĻµĮŠ“š°ø
Ļą¹ŲĢāÄæ



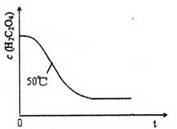
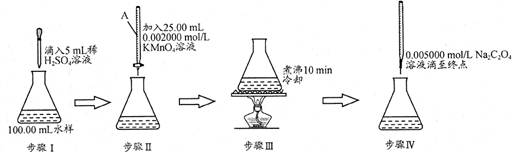
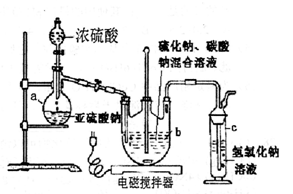
 =2I£+S4O
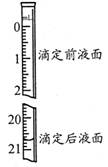
=2I£+S4O £¬µ±Ą¶É«ĶŹČ„H°ė·ÖÖÓ²»±äÉ«Ź±µ½“ļµĪ¶ØÖÕµć”£ŹµŃ鏿¾ŻČēĻĀ±ķ£ŗ
£¬µ±Ą¶É«ĶŹČ„H°ė·ÖÖÓ²»±äÉ«Ź±µ½“ļµĪ¶ØÖÕµć”£ŹµŃ鏿¾ŻČēĻĀ±ķ£ŗ