��Ŀ����
�Ƕ���ֽ⵰���ʵĹ��̿ɱ�ʾΪ����������1��30.0 mLţ���й��ж��ٿ˵���
��2������������к���16%������������ţ���к������ʵ����������Ƕ��٣�����֪ţ�̵��ܶ���
������(1)��ԭ�����ʵ�����n(N)=2n(H2SO4)-n(NaOH)=2��0.5 mol��L-1��
30 mLţ���к���ԭ�ӵ�������
(2)30 mLţ�̺��е����ʵ�����Ϊ![]() =
=
![]() ��100%=3.4%
��100%=3.4%
�𰸣�(1)

��ϰ��ϵ�д�
�����Ŀ
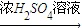 �����
�����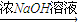 ��
��