��Ŀ����
�����ѹ滮������½����Ϻ�����Ȼ���ܵ����ߣ�ʵʩ���������䡱����1����Ȼ������Ҫ�ɷ���_______________�������ɶ���ֲ������ڸ�������������£���___________________�γɵġ�
��2����Ȼ�����Ǹ�Ч��Դ��Ҳ������������ԭ�ϣ�������Ȳ���������ȼ��顢�״�����ȩ�Ȼ�����Ʒ����д����Ȼ������Ҫ�ɷ־��������������·�Ӧ������Ȳ��̿�ڵĻ�ѧ����ʽ��_______________________��
��3����2005�꣬�ҹ�������������á��������䡱����Ȼ��������ȼ�ߵ����춼��Ҫ���졣��ͨ������˵����ͬ��ȼ��1 m3��ˮú������Ҫ�ɷ���CO��H2������Ȼ��������������ĸ��ࣿ
______________________________________________��
˼·��������Ȼ������Ҫ�ɷ��Ǽ��飬�����ɶ���ֲ������ڸ�������������£������·��ͺ�ֽ����õġ��������������Ӧ����Ψһ�ģ�����Ҳ���������ǣ���������ƽ�á�CO��H2ÿ1 mol���ĵ�������0.5 mol����1 mol��������Ҫ2 mol���������Խ�������Ҫ����
�𰸣���1������ ���·��ͺ�ֽ����õ�
��2��CH4![]() C+2H2��2CH4
C+2H2��2CH4![]() C2H2+3H2
C2H2+3H2
��3����Ȼ������������ࡣ

��ϰ��ϵ�д�
�����Ŀ
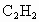 )��̿�ڵĻ�ѧ����ʽ��____________________��
)��̿�ڵĻ�ѧ����ʽ��____________________��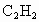 )��̿�ڵĻ�ѧ����ʽ��____________________��
)��̿�ڵĻ�ѧ����ʽ��____________________��