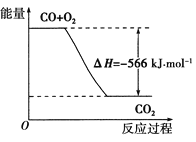
��Ŀ����
����Ŀ������ͼͼ���йص���������ȷ����

A. ��ʾ��mol H2 (g)��ȫȼ������ˮ��������241.8 kJ����
B. ��ʾ���Ȼ�ѧ����ʽΪ��H2(g)+ 1/2 O2(g) = H2O(g) ��H= ��241.8 kJ��mol-1
C. ��ʾ2 mol H2(g)�����е�����һ����2 mol��̬ˮ�����е�������483.6 kJ
D. H2O(g)����������H2(g)��O2(g)������֮��
���𰸡�B
��������
����A��2mol H2 (g)��ȫȼ������ˮ�����Ĺ������ڷ��ȷ�Ӧ���̣�ͼ���ʾ2mol H2 (g)��ȫȼ������ˮ�����ų�241.8 kJ����������B������ͼʾ����Ӧ������������ڲ�������������Ƿ��ȷ�Ӧ������ʽϵ�����룬�ʱ�Ҳ���룬�Ȼ�ѧ����ʽΪ��H2 (g)+1/2O2(g)�TH2O(g) ��H="-241.8" kJ/mol����ȷ��C����ʾ2mol H2 (g)��1mol O2 (g)�����е�������2 mol��̬ˮ�����е�������483.6 kJ������D��H2 (g)��O2 (g)��ȫȼ������ˮ�����Ĺ����Ƿ��ȵģ�1mol H2O(g)����������1mol H2 (g)��0.5mol O2 (g)�������ͣ�����

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д�
�����Ŀ