��Ŀ����
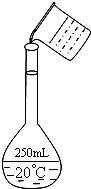 ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У����ձ� ��100mL��Ͳ ��500mL����ƿ ��ҩ�� �ݲ����� ��������ƽ�������룩
ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У����ձ� ��100mL��Ͳ ��500mL����ƿ ��ҩ�� �ݲ����� ��������ƽ�������룩��1������ʱ��û��ʹ�õ���������Ʒ��
��
��
������ţ�����ȱ�ٵ���������ͷ�ι�
��ͷ�ι�
����2�������Ƶ�ת�ƹ�����ijѧ����������ͼ��������ָ�����еĴ���
����ƿ�Ĺ�����
����ƿ�Ĺ�����
��δ�ò���������
δ�ò���������
��3������ʵ������������ȷ˳���ǣ�����ţ������ظ���
�٢ۢݢڢ�
�٢ۢݢڢ�
������������ƽ����10g NaOH�������С�ձ��У�����������ˮ�ܽ⣻
�ڼ���������ƿ�м�����ˮ��Һ���̶���1-2cm�������ý�ͷ�ι�С�ĵμ�����ˮ��
��Һ��Һ�����ʹ���̶������У�
�۰ѻָ����µ���ҺС�ĵ�ת��500mL����ƿ�У�
�ܽ�����ƿ���������ҡ�ȣ�
������������ˮϴ���ձ��벣����2-3�Σ�ϴ��Һһ��ת�Ƶ�����ƿ�У�
��4��������ƿʹ�÷����У����в�������ȷ���ǣ�����ţ�
C
C
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ����δ����
C�����������ƹ��������ƽ���̵���ֽ�ϣ�ȷ�����������ձ����ܽ������ע������ƿ��
D�����ݺ�����ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��תҡ��
��5��ʵ���������Ҫ2mol/L��NaOH��Һ950mL��������ʱӦѡ��
1000
1000
mL����ƿ����ȡNaOH��������80.0g
80.0g
����6���ڶ���ʱ������Ӧ��
�̶���
�̶���
������ƽ����������1������ʵ������IJ���ȷ�������������н��
��2������NaOH��Һ500mL��Ҫ��500mL����ƿ��������ƿ����Һ��Ҫ�ò�����������
��3������������Һ�IJ���������в���˳�������
��4��A����������跴���ߵ�ҡ�ȣ�
B����Һ�������ˮ���ݣ�����ƿ������ˮϴ����δ�����������ҺŨ����Ӱ�죻
C����������ϡ�ͷų��������ȣ���ȴ����������Һ�����ƫС��������ƿ���Ȳ��������ܵ�������Σ�գ�
D��ҡ��ʱʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��������ҡ�ȣ�
��5��ʵ����û��950mL����ƿ����Ӧѡ��1000mL����ƿ������n=cv������������Ƶ����ʵ������ٸ���m=nM���������������Ƶ�������
��6���ڶ���ʱ������Ӧ��̶��߱�����ƽ��
��2������NaOH��Һ500mL��Ҫ��500mL����ƿ��������ƿ����Һ��Ҫ�ò�����������
��3������������Һ�IJ���������в���˳�������
��4��A����������跴���ߵ�ҡ�ȣ�
B����Һ�������ˮ���ݣ�����ƿ������ˮϴ����δ�����������ҺŨ����Ӱ�죻
C����������ϡ�ͷų��������ȣ���ȴ����������Һ�����ƫС��������ƿ���Ȳ��������ܵ�������Σ�գ�
D��ҡ��ʱʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��������ҡ�ȣ�
��5��ʵ����û��950mL����ƿ����Ӧѡ��1000mL����ƿ������n=cv������������Ƶ����ʵ������ٸ���m=nM���������������Ƶ�������
��6���ڶ���ʱ������Ӧ��̶��߱�����ƽ��
�����1�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬���ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�������Ҫ�������У���ƽ���ձ�����������500mL����ƿ����ͷ�ιܡ�ҩ�ף�
�ʿ��Բ�ʹ�õ�����Ϊ����100mL��Ͳ
��ȱ��Ҫ�������У���ͷ�ιܣ�
�ʴ�Ϊ���ڣ���ͷ�ιܣ�
��2������NaOH��Һ500mL��Ҫ��500mL����ƿ��������ƿ����Һ��Ҫ�ò�����������
�ʴ�Ϊ������ƿ�Ĺ�����δ�ò�����������
��3���ɣ�1�����������֪����ȷ˳���Ǣ٢ۢݢڢܣ�
�ʴ�Ϊ���٢ۢݢڢܣ�
��4����4��A����������跴���ߵ�ҡ�ȣ���ʹ������ƿǰ������Ƿ�©ˮ����A��ȷ��
B����Һ�������ˮ���ݣ�����ƿ������ˮϴ����δ�����������ҺŨ����Ӱ�죬��B��ȷ��
C����������ϡ�ͷų��������ȣ���ȴ����������Һ�����ƫС��������ƿ���Ȳ��������ܵ�������Σ�գ�����������ƿ���ܽ��������ƣ���C����
D��ҡ��ʱʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��������ҡ�ȣ���D��ȷ��
��ѡC��
��5��ʵ����û��950mL����ƿ����Ӧѡ��1000mL����ƿ�����������Ƶ�����Ϊ1L��2mol?L-1��40g/mol=80.0g��
�ʴ�Ϊ��1000��80.0g��
��6���ڶ���ʱ������Ӧ��̶��߱�����ƽ��
�ʴ�Ϊ���̶��ߣ�
�ʿ��Բ�ʹ�õ�����Ϊ����100mL��Ͳ
��ȱ��Ҫ�������У���ͷ�ιܣ�
�ʴ�Ϊ���ڣ���ͷ�ιܣ�
��2������NaOH��Һ500mL��Ҫ��500mL����ƿ��������ƿ����Һ��Ҫ�ò�����������
�ʴ�Ϊ������ƿ�Ĺ�����δ�ò�����������
��3���ɣ�1�����������֪����ȷ˳���Ǣ٢ۢݢڢܣ�
�ʴ�Ϊ���٢ۢݢڢܣ�
��4����4��A����������跴���ߵ�ҡ�ȣ���ʹ������ƿǰ������Ƿ�©ˮ����A��ȷ��
B����Һ�������ˮ���ݣ�����ƿ������ˮϴ����δ�����������ҺŨ����Ӱ�죬��B��ȷ��
C����������ϡ�ͷų��������ȣ���ȴ����������Һ�����ƫС��������ƿ���Ȳ��������ܵ�������Σ�գ�����������ƿ���ܽ��������ƣ���C����
D��ҡ��ʱʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��������ҡ�ȣ���D��ȷ��
��ѡC��
��5��ʵ����û��950mL����ƿ����Ӧѡ��1000mL����ƿ�����������Ƶ�����Ϊ1L��2mol?L-1��40g/mol=80.0g��
�ʴ�Ϊ��1000��80.0g��
��6���ڶ���ʱ������Ӧ��̶��߱�����ƽ��
�ʴ�Ϊ���̶��ߣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ��Ѷ��еȣ�����c=
������Һ����ԭ������������ע���������ƾ��и�ʴ�ԡ��׳��⣬Ӧ���ձ���Ѹ�ٳ�����
| n |
| V |

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У�
ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У�
