��Ŀ����
��ʱ��/s Ũ�� mol��L-1 | 0 | 20 | 40 | 60 | 80 | 100 |
c(N2O4)/mol��L-1 | 0.100 | c1 | 0.050 | c3 | a | b |
c(NO2)/mol��L-1 | 0.000 | 0.060 | c2 | 0.120 | 0.120 | 0.120 |
����գ�
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ______________________________���ﵽƽ��ʱ������������ת����Ϊ__________%������c2__________c3__________ab__________(ѡ�������������=��)��
(2)20 sʱ������������Ũ��c1=_________mol��L-1����0��20 s��������������ƽ����Ӧ����Ϊ________________mol��(L��s)-1��
(3)������ͬ���������������������Ƕ����������壬Ҫ�ﵽ����ͬ����ƽ��״̬��������������ʼŨ����______________mol��L-1��
(1)N2O4![]() 2NO2(��д������ţ�����ƽ����) 60 �� = =
2NO2(��д������ţ�����ƽ����) 60 �� = =
(2)0.070 0.0015
(3)0.200
������(1)��Ϊ60 s��80 s��100 s��NO2��Ũ�Ⱦ�Ϊ0.120 mol��L-1��˵���Ѵ�ƽ�⣬��N2O4�ķֽⷴӦΪ���淴Ӧ��
N2O4 ![]() 2NO2
2NO2
��ʼŨ�� 0.100 0
�仯Ũ�� 0.060 0.120
ƽ��Ũ�� 0.040 0.120
����������ת����Ϊ![]() ��100%=60%
��100%=60%
c2=
(2)c1=0.100 mol��L-1-0.030 mol��L-1=0.070 mol��L-1
v(N2O4)=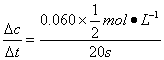 =0.001 5 mol��L-1��s-1
=0.001 5 mol��L-1��s-1
(3)����ͬ������������������г����NO2���壬���ݷ���ʽ��ѧ������������N2O4Ũ�ȵ�2������0.200 mol��L-1��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A����ƿ��������ܶȲ��ٱ仯 | B����ƿ���������ɫ���ٱ仯 | C����ƿ�������ѹǿ���ٱ仯 | D����ƿ�������ƽ����Է����������ٱ仯 |

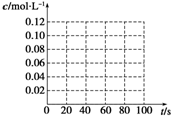 ��100��ʱ����0.40mol�Ķ��������������2L��յ��ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ����������ʾ��
��100��ʱ����0.40mol�Ķ��������������2L��յ��ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ����������ʾ��