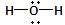��Ŀ����
��14�֣�������̼����̼���������нϸߵ����ú�ҵ��ֵ���ڶ����������Ź㷺��Ӧ�á�Ŀǰ��������̼��������ʡ��߲ˣ����ࣩ���ʡ������ɽ������ϵ�����Ҳչ�����õķ�չǰ����������̼��һ����ɫ��ζ�����壬���������粢��û�п�ȼ�ԡ����ǽ���þ�ڵ�ȼ�������¿����ڶ�����̼������ȼ�ա����л�ԭ������̼��
������������
����д����ѧ��Ӧ����ʽ������˫���ŷ���ʾ�÷�Ӧ�ĵ���ת���� ��
��
��CO2���������Һ��Ӧʱ��������ͬ�����ɵIJ��ﲻͬ��
ȡ���ݵ����ʵ���Ũ�ȵ������Ca(OH)2����Һ��һ��ͨ�����CO2�����ɳ��������ʵ�����n����ͨ��CO2�����V���Ĺ�ϵ��ͼ��ʾ


д�������仯��a��b�����ӷ���ʽ��
����һ���ȼ���������KOH�����ܽ⣬�ٽ�����CO2ͨ��KOH��Ca(OH)2�Ļ����Һ�У����ͼ��ʾ�����ɳ��������ʵ�����n����ͨ��CO2�����V���Ĺ�ϵ;
 ��д������ͼ�в�ͬ���ߴ���Ӧ�����ӷ�Ӧ����ʽ ��
��д������ͼ�в�ͬ���ߴ���Ӧ�����ӷ�Ӧ����ʽ ��
��
������������
����д����ѧ��Ӧ����ʽ������˫���ŷ���ʾ�÷�Ӧ�ĵ���ת����
 ��
�� ��CO2���������Һ��Ӧʱ��������ͬ�����ɵIJ��ﲻͬ��
ȡ���ݵ����ʵ���Ũ�ȵ������Ca(OH)2����Һ��һ��ͨ�����CO2�����ɳ��������ʵ�����n����ͨ��CO2�����V���Ĺ�ϵ��ͼ��ʾ


д�������仯��a��b�����ӷ���ʽ��
����һ���ȼ���������KOH�����ܽ⣬�ٽ�����CO2ͨ��KOH��Ca(OH)2�Ļ����Һ�У����ͼ��ʾ�����ɳ��������ʵ�����n����ͨ��CO2�����V���Ĺ�ϵ;
 ��д������ͼ�в�ͬ���ߴ���Ӧ�����ӷ�Ӧ����ʽ ��
��д������ͼ�в�ͬ���ߴ���Ӧ�����ӷ�Ӧ����ʽ ����
��14�֣���MgO (2��)
��

����ȷд������ʽ����ƽ��ȷ��
 1
1 �֣����ű�ע��ȷ��2�֣�����3�֣�
�֣����ű�ע��ȷ��2�֣�����3�֣���
 CaCO3 + CO2 + H2O = Ca2+ + 2HCO3- (2��)
CaCO3 + CO2 + H2O = Ca2+ + 2HCO3- (2��)��
|
 CO2 + 2OH- = CO32- + H2O (2��)
CO2 + 2OH- = CO32- + H2O (2��)CO32- +CO2 + H2O = 2HCO3- (2��)
��

��ϰ��ϵ�д�
 �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�
�����Ŀ

 ���ɵ�·�Ļ������ϡ���ѧ��Ԥ�ƣ���2011��һ������оƬ�Ͻ��Ἧ��10�ڸ�����
���ɵ�·�Ļ������ϡ���ѧ��Ԥ�ƣ���2011��һ������оƬ�Ͻ��Ἧ��10�ڸ����� �ܣ��书��Զ�����������Ҫ��Ķ࣬��Թ�Ĵ���Ҫ��ܸߡ��û�ѧ�������Ƶøߴ��ȹ裬�仯ѧ����ʽΪ�� ��SiO2 + 2C
�ܣ��书��Զ�����������Ҫ��Ķ࣬��Թ�Ĵ���Ҫ��ܸߡ��û�ѧ�������Ƶøߴ��ȹ裬�仯ѧ����ʽΪ�� ��SiO2 + 2C  Si + 2CO ��Si + 2Cl2
Si + 2CO ��Si + 2Cl2 SiCl4
SiCl4 Si + 4HCl���ش��������⣺
Si + 4HCl���ش��������⣺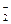 + xI-+ yH+ = 2NO��+ I2 + zH2O����ش��������⣺
+ xI-+ yH+ = 2NO��+ I2 + zH2O����ش��������⣺