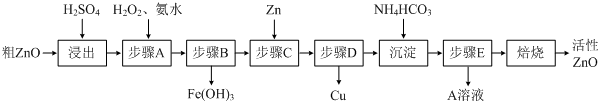
��Ŀ����
����ZnO�������ϡ�Ϳ�Ϲ�ҵ����ҪӦ�ã��ɴ�ZnO����FeO��CuO���Ʊ�����ZnO������ͼ��

��1�����������У������õ����ܶ�Ϊ1.5g/cm3����������Ϊ60%H2SO4�����H2SO4�����ʵ���Ũ��Ϊ
��2����֪��������Fe��OH��3��Ksp=4.0��10-38����������H2SO4��������Һ��Fe3+��Ũ��Ϊ0.04mol/L��������Һ��pH������H2O2������������ɫ������
����Һ��pH����Ϊ
����������Һ��pH�����ܵ��µĺ����
��3��A����Ҫ���е�������
��4����֪���Ʊ�����ZnO�������յ������Ǽ�ʽ̼��п�����ܺ��ᾧˮ����ȡ��ʽ̼��п6.82g������HCl����CO2 448mL����״���£����ܽ��������HCl 0.12mol�����Ʋ�ü�ʽ̼��п�Ļ�ѧʽ

��1�����������У������õ����ܶ�Ϊ1.5g/cm3����������Ϊ60%H2SO4�����H2SO4�����ʵ���Ũ��Ϊ
9.18mol/L
9.18mol/L
����������λС������2����֪��������Fe��OH��3��Ksp=4.0��10-38����������H2SO4��������Һ��Fe3+��Ũ��Ϊ0.04mol/L��������Һ��pH������H2O2������������ɫ������
����Һ��pH����Ϊ
2
2
ʱ����Һ��Fe3+��ʼ����������������Һ��pH�����ܵ��µĺ����
����Cu��OH��2��Zn��OH��2����
����Cu��OH��2��Zn��OH��2����
����3��A����Ҫ���е�������
��NH4��2SO4
��NH4��2SO4
����4����֪���Ʊ�����ZnO�������յ������Ǽ�ʽ̼��п�����ܺ��ᾧˮ����ȡ��ʽ̼��п6.82g������HCl����CO2 448mL����״���£����ܽ��������HCl 0.12mol�����Ʋ�ü�ʽ̼��п�Ļ�ѧʽ
Zn3��OH��4CO3?H2O
Zn3��OH��4CO3?H2O
����������1������Ũ����ϡ��ǰ�����ʵ����������Ũ����������
��2�����ݳ����ܽ�ƽ���е��ܶȻ���������������Ũ�ȣ������Һ�����ӻ���������õ�������Ũ�ȼ�����ҺpH��
��3���������̷�����֪��̼�����ϴ�ӳ����������ᷴӦ��������泥�����A����Ҫ���е������ǣ�NH4��2SO4��
��4������Ԫ���غ��ϵ�ֱ����������ʵ���������������ɵ����ӹ�ϵ����Ӧ�Ķ�����ϵ��������ϵ����õ��жϳ��ĸ��ɷ����ʵ���ȡ��������ȵõ���ѧʽ��
��2�����ݳ����ܽ�ƽ���е��ܶȻ���������������Ũ�ȣ������Һ�����ӻ���������õ�������Ũ�ȼ�����ҺpH��
��3���������̷�����֪��̼�����ϴ�ӳ����������ᷴӦ��������泥�����A����Ҫ���е������ǣ�NH4��2SO4��
��4������Ԫ���غ��ϵ�ֱ����������ʵ���������������ɵ����ӹ�ϵ����Ӧ�Ķ�����ϵ��������ϵ����õ��жϳ��ĸ��ɷ����ʵ���ȡ��������ȵõ���ѧʽ��
����⣺��1��ϡ��������ʵ���Ũ��=
=
=9.18mol/L���ʴ�Ϊ��9.18mol/L��
��2������֪��������Fe��OH��3��Ksp=4.0��10-38����������H2SO4��������Һ��Fe3+��Ũ��Ϊ0.04mol/L��������Һ��pH������H2O2������������ɫ������
������������������ʱ����Һ��Ksp=c3��OH-��c��Fe3+��=4.0��10-38 c��OH-��=1��10-12mol/L��c��H+��=10-2mol/L��pH=2��
�ʴ�Ϊ��2��
����������Һ��pH�����ܵ��µĺ����ͭ���Ӻ�п���ӳ�����
�ʴ�Ϊ������Cu��OH��2��Zn��OH��2������
��3���������̷�����̼�����ϴ�ӳ����������ᷴӦ��������泥�����A����Ҫ���е������ǣ�NH4��2SO4���ʴ�Ϊ����NH4��2SO4��
��4��n��CO32-��=n��CO2��=0.02 mol��
n��Zn2+��=n��ZnCl2��=
n��Cl-��=
n��HCl��=0.06 mol��
n��OH-��=2n��Zn2+��-2n��CO32-��=0.08 mol��
n��H2O��=[6.82 g-n��Zn2+��-n��OH-��-n��CO32-��]��18 g/mol=0.02 mol��
n��Zn2+����n��OH-����n��CO32-����n��H2O��=0.06��0.08��0.02��0.02=3��4��1��1��
��ѧʽΪ��Zn3��OH��4CO3?H2O��
�ʻ�ѧʽΪ��Zn3��OH��4CO3?H2O��
| 1000��w |
| M |
| 1000��1.5��60% |
| 98 |
��2������֪��������Fe��OH��3��Ksp=4.0��10-38����������H2SO4��������Һ��Fe3+��Ũ��Ϊ0.04mol/L��������Һ��pH������H2O2������������ɫ������
������������������ʱ����Һ��Ksp=c3��OH-��c��Fe3+��=4.0��10-38 c��OH-��=1��10-12mol/L��c��H+��=10-2mol/L��pH=2��
�ʴ�Ϊ��2��
����������Һ��pH�����ܵ��µĺ����ͭ���Ӻ�п���ӳ�����
�ʴ�Ϊ������Cu��OH��2��Zn��OH��2������
��3���������̷�����̼�����ϴ�ӳ����������ᷴӦ��������泥�����A����Ҫ���е������ǣ�NH4��2SO4���ʴ�Ϊ����NH4��2SO4��
��4��n��CO32-��=n��CO2��=0.02 mol��
n��Zn2+��=n��ZnCl2��=
| 1 |
| 2 |
| 1 |
| 2 |
n��OH-��=2n��Zn2+��-2n��CO32-��=0.08 mol��
n��H2O��=[6.82 g-n��Zn2+��-n��OH-��-n��CO32-��]��18 g/mol=0.02 mol��
n��Zn2+����n��OH-����n��CO32-����n��H2O��=0.06��0.08��0.02��0.02=3��4��1��1��
��ѧʽΪ��Zn3��OH��4CO3?H2O��
�ʻ�ѧʽΪ��Zn3��OH��4CO3?H2O��
���������⿼��һ�����ʵ���Ũ�Ȼ�����㡢�ܶȻ��������йؼ��㡢��ѧʽ��ȷ����֪ʶ�㣬�����Ԫ���غ�������ʻ�ѧʽ���Ѷ��еȣ�

��ϰ��ϵ�д�
 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
�����Ŀ
��15�֣�����ZnO�������ϡ�Ϳ�Ϲ�ҵ������ҪӦ�ã�һ���ɴ�ZnO����FeO��CuO���Ʊ�����ZnO���������£���֪����ʽ̼��п�����տ��Ƶû���ZnO����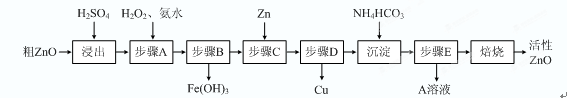
��֪�������������������������ʱ��pH���±���
| ���������� | Fe2�� | Fe3�� | Zn2�� | Cu2�� |
| ��ʼ����ʱpH | 6.34 | 1.48 | 6.2 | 5.2 |
| ��ȫ����ʱpH | 9.7 | 3.2 | 8.0 | 6.4 |
�Ų���A��H2O2������Ӧ�����ӷ���ʽ�� ���ò����������ҺpH�ķ�Χ�� ��
�� A��Һ����Ҫ���е������� ��
�Ǽ�ʽ̼��п�������Ƶû���ZnO�ķ�Ӧ��H��0���÷�Ӧ���Է����е�ԭ���� ��
������������ķ�ˮpH��8����ʱZn2����Ũ��Ϊ mg/L�������£�Ksp[Zn(OH)2]��1.2��10-17����