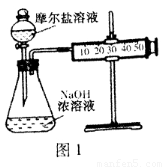
��Ŀ����
��1��ʵ���������������ۺ�ϡ������ȡ��������������Һ��Ϊ��ֹ����ʣ�Ӧ�ڸ���Һ��Ӧ��������______��
��2�����Ƶ��̷����壨FeSO4?7H2O����dz��ɫ�ģ����ڿ����м��ױ�ɻ�ɫ������ɫ�ļ�ʽ������[Fe��OH��SO4]��д���÷�Ӧ�Ļ�ѧ����ʽ��______��
��3����֪FeSO4�ڲ�ͬ�����·ֽ�õ��IJ��ﲻͬ��������FeO��SO3��Ҳ������Fe2O3��SO3��SO2��ij�о�С��̽���ھƾ���Ƽ���������FeSO4�ֽ����������֪SO3���۵���16.8��C���е���44.8��C��
��װ��II���Թ��в�װ�κ��Լ�����������______���Թܽ�����50�����ˮԡ�У�Ŀ����______��
��װ��III��װ��IV��������̽����ʵ���������ɷ֣������ʵ����ƣ���д�����Լ���Ԥ����������ۣ�
��ѡ�Լ���3mol��L-1��H2S04��6mol��L-l NaOH��0.5mol��L -1 BaCl2��O.5mol��L-1Ba��NO3��2��0.01mol��L-1 ���� KMnO4 ��Һ��0.0l mol��L-1 ��ˮ��
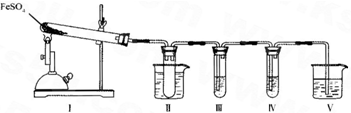
| �����Լ� | Ԥ����������� |
| װ��III���Թ��м�������______�� | ����������ɫ������֤����������к���SO3�� |
| װ��IV���Թ��м�������______�� | ______ ______�� |
����μ���װ�â��й�����ȫ�ֽ�����ɹ����������FeO��д�����衢�����ۣ�______��
��2���������Ӿ��л�ԭ�ԣ��ױ�������������Ӧ����ʽΪ��4FeSO4?7H2O+O2=4Fe��OH��SO4+5H2O���ʴ�Ϊ��4FeSO4?7H2O+O2=4Fe��OH��SO4+5H2O��
��3���ٲ��������弫����Һ��Ӧʱ�����������������װ��II���Թ��ܷ�ֹ��Һ������װ�â��У���ȫƿ����SO3�ķе���44.8��C�����¶ȸ���44.8��Cʱ��������Ϊ����״̬�����Թܽ�����50�����ˮԡ���ܷ�ֹSO3Һ�������̣��ʴ�Ϊ����ֹ��Һ������װ�â��У���ȫƿ������ֹSO3Һ�������̣�
��FeSO4�ֽ������������Ϊ��������Ҳ����Ϊ��������Ͷ�������Ļ������������������������ɰ�ɫ����������������ʹ������ػ���ˮ��ɫ���ʴ�Ϊ��
| 0.5 mol��L -1 BaCl2 | |
| 0.01 mol��L-1 ���� KMnO4 ��Һ����0.0l mol��L-1 ��ˮ�� | ����Һ��ɫ�����ɫ����ȥ��֤����������к���SO2������Һ��ɫ�����ɫ�������Ա仯��֤����������в���SO2 |
��FeO�е���Ϊ+2�ۣ����������Ὣ�������ܽ⣬�õ�������������ʹ���������ɫ���ʴ�Ϊ��ȡ�����ֽ��ʣ��������Թ��У����Թ��м�������ϡ���ᣬ��ַ�Ӧ��������KMnO4��Һ��������KMnO4��Һ��ɫ��˵�����ɵĹ�����ﺬ��FeO��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д����������DZȽ���Ҫ�������Σ���ũҵ������ũҩ������С����벡���ڹ�ҵ������Ⱦɫ����������īˮ��ľ�ķ��������ݼ��ȣ�
��1��ʵ���������������ۺ�ϡ������ȡ��������������Һ��Ϊ��ֹ����ʣ�Ӧ�ڸ���Һ��Ӧ��������______��
��2�����Ƶ��̷����壨FeSO4?7H2O����dz��ɫ�ģ����ڿ����м��ױ�ɻ�ɫ������ɫ�ļ�ʽ������[Fe��OH��SO4]��д���÷�Ӧ�Ļ�ѧ����ʽ��______��
��3����֪FeSO4�ڲ�ͬ�����·ֽ�õ��IJ��ﲻͬ��������FeO��SO3��Ҳ������Fe2O3��SO3��SO2��ij�о�С��̽���ھƾ���Ƽ���������FeSO4�ֽ����������֪SO3���۵���16.8��C���е���44.8��C��
��װ��II���Թ��в�װ�κ��Լ�����������______���Թܽ�����50�����ˮԡ�У�Ŀ����______��
��װ��III��װ��IV��������̽����ʵ���������ɷ֣������ʵ����ƣ���д�����Լ���Ԥ����������ۣ�
��ѡ�Լ���3mol��L-1��H2S04��6mol��L-1 NaOH��0.5mol��L -1 BaCl2��O.5mol��L-1Ba��NO3��2��0.01mol��L-1 ���� KMnO4 ��Һ��0.0l mol��L-1 ��ˮ��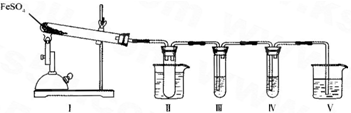
| �����Լ� | Ԥ����������� |
| װ��III���Թ��м�������______�� | ����������ɫ������֤����������к���SO3�� |
| װ��IV���Թ��м�������______�� | ______ ______�� |
��װ��V�������Ƿ�ֹβ����Ⱦ�������ձ���Ӧ������Լ���______��
����μ���װ�â��й�����ȫ�ֽ�����ɹ����������FeO��д�����衢�����ۣ�______��
��1��ʵ���������������ۺ�ϡ������ȡ��������������Һ��Ϊ��ֹ����ʣ�Ӧ�ڸ���Һ��Ӧ��������______��
��2�����Ƶ��̷�������dz��ɫ�ģ����ڿ����м��ױ�ɻ�ɫ������ɫ�ļ�ʽ������[Fe��OH��SO4]��д���÷�Ӧ�Ļ�ѧ����ʽ��______��
��3����֪FeSO4�ڲ�ͬ�����·ֽ�õ��IJ��ﲻͬ��������FeO��SO3��Ҳ������Fe2O3��SO3��SO2��ij�о�С��̽���ھƾ���Ƽ���������FeSO4�ֽ����������֪SO3���۵���16.8°C���е���44.8°C��
��װ��II���Թ��в�װ�κ��Լ�����������______���Թܽ�����50�����ˮԡ�У�Ŀ����______��
��װ��III��װ��IV��������̽����ʵ���������ɷ֣������ʵ����ƣ���д�����Լ���Ԥ����������ۣ�
��ѡ�Լ���3mol��L-1��H2S04��6mol��L-l NaOH��0.5mol��L -1 BaCl2��O.5mol��L-1Ba��NO3��2��0.01mol��L-1 ���� KMnO4 ��Һ��0.0l mol��L-1 ��ˮ��
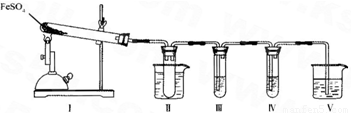
| �����Լ� | Ԥ����������� |
| װ��III���Թ��м�������______�� | ����������ɫ������֤����������к���SO3�� |
| װ��IV���Թ��м�������______�� | ______ ______�� |
����μ���װ�â��й�����ȫ�ֽ�����ɹ����������FeO��д�����衢�����ۣ�______��