��Ŀ����
��һ���¶��½�2mol A��2mol B������������2L�ܱ������У��������·�Ӧ��3A(g)��B(g)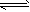 2C(g)
2C(g)
��2D(g)��2����ĩ��Ӧ�ﵽƽ��״̬��������0.8mol D������д����հס�
��1����D��ʾ��ƽ����Ӧ����Ϊ____________A��ת����Ϊ____________��
��2�������С�����ݻ�(�¶Ȳ���)����ƽ����ϵ�л��������ܶ�____________(����������١����䡱)��
��3������ʼʱֻ��C��D��4/3mol��Ҫʹƽ��ʱ�����ʵ�����������ԭƽ����ȣ���Ӧ����_______mol
B���ʡ�
��4������ԭƽ����ϵ��Ͷ��1mol A��1mol B��ƽ��____________(����ơ����ƻ��ơ�)��
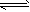 2C(g)
2C(g)��2D(g)��2����ĩ��Ӧ�ﵽƽ��״̬��������0.8mol D������д����հס�
��1����D��ʾ��ƽ����Ӧ����Ϊ____________A��ת����Ϊ____________��
��2�������С�����ݻ�(�¶Ȳ���)����ƽ����ϵ�л��������ܶ�____________(����������١����䡱)��
��3������ʼʱֻ��C��D��4/3mol��Ҫʹƽ��ʱ�����ʵ�����������ԭƽ����ȣ���Ӧ����_______mol
B���ʡ�
��4������ԭƽ����ϵ��Ͷ��1mol A��1mol B��ƽ��____________(����ơ����ƻ��ơ�)��
��1��0.2 mol��L-1��min-1��60%
��2������
��3��4/3
��4������
��2������
��3��4/3
��4������

��ϰ��ϵ�д�
�����Ŀ