��Ŀ����
������ȫ�����ǵ¹���ȫ����Ҫ���ϡ�������������ײ��˲�䣬��ȫװ��ͨ����ʹ���еķ�ĩ�ֽ��ͷų������ĵ����γ����ң��Ӷ�����˾�����˿������˺���Ϊ�о���ȫ���ҹ����Ļ�ѧԭ����ȡ��ȫװ���еķ�ĩ����ʵ�顣����ɷ�����ȷ���÷�ĩ����Na��Fe��N��O����Ԫ�ء�ˮ������������������ĩ�����ܽ⡣����⣬������Ϊ������ף�������Ϊ����ɫ���壬���������ᡣȡ13.0 g������ף�����ʹ����ȫ�ֽ⣬���ɵ����͵����ң����ɵĵ����ۺϳɱ�״���µ����Ϊ6.72 L���������ڸ��¸����������������벻�������ɫ��ĩ��Ӧ���ɻ������������һ�ֵ��ʡ��������������Ӵ���ת��Ϊ�������Ρ���ش��������⣺
(1)�Ļ�ѧʽΪ_________�����ĵ���ʽΪ__________
(2)�����ڿ�����ת��Ϊ̼�����Σ���Ӧ�Ļ�ѧ����ʽΪ____________________
(3)�����������ɫ��ĩ������Ӧ�Ļ�ѧ����ʽΪ_______________________����ȫ�����к���ɫ��ĩ��������_________________
(4)���������У��п�����Ϊ��ȫ�����к���ɫ��ĩ���Ʒ����____��
A��KCl B��KNO3 C��Na2S D��CuO
(5)���һ��ʵ�鷽����̽���������������Ӵ������ɿ������εijɷ֣������ǽᾧˮ���
___________________��
(1)�Ļ�ѧʽΪ_________�����ĵ���ʽΪ__________
(2)�����ڿ�����ת��Ϊ̼�����Σ���Ӧ�Ļ�ѧ����ʽΪ____________________
(3)�����������ɫ��ĩ������Ӧ�Ļ�ѧ����ʽΪ_______________________����ȫ�����к���ɫ��ĩ��������_________________
(4)���������У��п�����Ϊ��ȫ�����к���ɫ��ĩ���Ʒ����____��
A��KCl B��KNO3 C��Na2S D��CuO
(5)���һ��ʵ�鷽����̽���������������Ӵ������ɿ������εijɷ֣������ǽᾧˮ���
___________________��
(1)Na3N��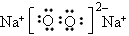
(2)2Na2O2+4CO2+2H2O=4NaHCO3+O2
(3)6Na+2Fe2O3=3Na2O2+4Fe���䵱����������ȥ�����Ʒֽ�����Ľ����ƣ�����������ˮ�����������Ⱥͼ����к����ʣ��ṩ�������������ڵ����Ƶ�Ѹ�ٷֽ�
(4)D
(5)ʵ��Ŀ�ģ�����Na2O2�ڿ�������ˮ�������̼��Ӧ�IJ��������NaOH��Na2CO3��NaHCO3��
ʵ�����һ��
ʵ��ԭ����������������
���裺�ٳ�����Ϲ�����������ڽ��������ȣ���������ͨ�����ʯ��ˮ������������NaHCO3��ʯ��ˮ����ǣ�����NaHCO3��NaOH������ʯ��ˮ�����仯��mg���ۼ���������ᣬ������������ͨ�����ʯ��ˮ������ʯ��ˮ����������ng����ͨ��m��n �ļ��������ս����
ʵ����ƶ���
ʵ��ԭ�����ⶨ�������������������̼�����Ĺ�ϵȷ�������ɷ֡�
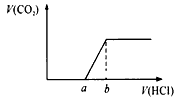
�ٲ�����������̼������ֻ�� NaOH���ڿ�ʼ����������̼ǰ���뿪ʼ����������ֱ̼����������ĵ�����������Ϊ1��1����ֻ��Na2CO3������1��1 ��ΪNaOH��Na2CO3�Ļ���С��1��1��Ϊ
Na2CO3��NaHCO3�Ļ������Ƚ���ͼ��a��b�Ĵ�С��
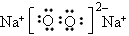
(2)2Na2O2+4CO2+2H2O=4NaHCO3+O2
(3)6Na+2Fe2O3=3Na2O2+4Fe���䵱����������ȥ�����Ʒֽ�����Ľ����ƣ�����������ˮ�����������Ⱥͼ����к����ʣ��ṩ�������������ڵ����Ƶ�Ѹ�ٷֽ�
(4)D
(5)ʵ��Ŀ�ģ�����Na2O2�ڿ�������ˮ�������̼��Ӧ�IJ��������NaOH��Na2CO3��NaHCO3��
ʵ�����һ��
ʵ��ԭ����������������
���裺�ٳ�����Ϲ�����������ڽ��������ȣ���������ͨ�����ʯ��ˮ������������NaHCO3��ʯ��ˮ����ǣ�����NaHCO3��NaOH������ʯ��ˮ�����仯��mg���ۼ���������ᣬ������������ͨ�����ʯ��ˮ������ʯ��ˮ����������ng����ͨ��m��n �ļ��������ս����
ʵ����ƶ���
ʵ��ԭ�����ⶨ�������������������̼�����Ĺ�ϵȷ�������ɷ֡�
�ٲ�����������̼������ֻ�� NaOH���ڿ�ʼ����������̼ǰ���뿪ʼ����������ֱ̼����������ĵ�����������Ϊ1��1����ֻ��Na2CO3������1��1 ��ΪNaOH��Na2CO3�Ļ���С��1��1��Ϊ
Na2CO3��NaHCO3�Ļ������Ƚ���ͼ��a��b�Ĵ�С��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ