��Ŀ����
 ��2011?����ģ�⣩��A��B��C��D��E��F���ֶ�����Ԫ�أ���Ԫ��������Ϣ���±���
��2011?����ģ�⣩��A��B��C��D��E��F���ֶ�����Ԫ�أ���Ԫ��������Ϣ���±���| Ԫ�ر�� | Ԫ��������Ϣ |
| A | A�ĵ������ܶ���С������ |
| B | B�ĵ���������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ������������ |
| C | C��ԭ�����������������ڲ������������ |
| D | D��Bͬ���ڣ���������D�ļ����Ӱ뾶��С |
| E | B��C��E��ɵ�36���ӵĻ�����Y�Ǽ�������������Ҫ�ɷ� |
| F | FԪ���������������۵Ĵ�����Ϊ4 |
���Ӽ������ۼ�
���Ӽ������ۼ�
����2��D��E��F�ļ����Ӱ뾶�ɴ�С��˳���ǣ��û�ѧʽ��ʾ��
S2-��Cl-��Al3+
S2-��Cl-��Al3+
����3��д�����־���A��B��C��F����Ԫ�صĻ���������Һ�����Ӧ�����ӷ���ʽ
H++HSO-3=SO2��+H2O
H++HSO-3=SO2��+H2O
����4����Fe��D������ɵĻ�����У���������F������������Ӧˮ�����ϡ��Һ������ȫ���ܽ⣮�����õ���Һ�м������������������Һ���������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ���ԭ�������D���ʵ���������Ϊ
30%
30%
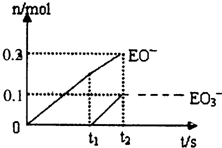
����5��һ������ʯ������ͨ��һ������E���ʣ�����ǡ����ȫ��Ӧ���������������ֺ�EԪ�ص����ӣ������������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ����t2ʱ������������������Ϊ
37g
37g
g����ʱ��Ӧ�Ļ�ѧ����ʽΪ10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O
10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O
����6��A��B�γɵĻ�����BA���л��ϳ�����;�ܹ㷺�������Զ�ȡ�ܶ�����е����Ӷ�������Ӧ���ƻ����д�������Ҵ���Ӧ�Ļ�ѧ����ʽ
NaH+CH3CH2OH=CH3CH2ONa+H2��
NaH+CH3CH2OH=CH3CH2ONa+H2��
��������A�ĵ������ܶ���С�����ʣ���A����Ԫ�أ�
B�ĵ���������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ�����������ӣ���B�Ƕ�����Ԫ�أ�����B����Ԫ�أ�
C��ԭ�����������������ڲ����������������C�Ƕ�����Ԫ�أ�����C����Ԫ�أ�
D��Bͬ���ڣ���������D�ļ����Ӱ뾶��С����D����Ԫ�أ�
B��C��E��ɵ�36���ӵĻ�����Y�Ǽ�������������Ҫ�ɷ֣�����������Ҫ�ɷ��Ǵ������ƣ�����E����Ԫ�أ�
FԪ���������������۵Ĵ�����Ϊ4����F�Ƕ�����Ԫ�أ�����F����Ԫ�أ�
��1�����ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ����ǽ���Ԫ��֮�����γɹ��ۼ���
��2�����Ӳ���Խ������ӣ���뾶Խ���Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ���������������С��
��3������A��B��C��F����Ԫ�صĻ��������������ƺ����������ƣ��������Ƴ�ǿ�����ܺ����������Ʒ�Ӧ��
��4���������Ļ�����������ϡ�����������������������������������������������Һ�м����������������Һ����������������������ƫ��������Һ��������ϴ�ӡ�������պ�õ�һ�ֹ����������������������������������Ļ����������ȣ��������������൱����Ԫ�ص����������������������㼴�ɣ�
��5�����ݵ�ʧ�������д����Ӧ�Ļ�ѧ��Ӧ����ʽ�����ݷ�Ӧ����ʽ�����������Ƶ�������
��6���⻯�����Ҵ���Ӧ�����Ҵ��ƺ�������
B�ĵ���������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ�����������ӣ���B�Ƕ�����Ԫ�أ�����B����Ԫ�أ�
C��ԭ�����������������ڲ����������������C�Ƕ�����Ԫ�أ�����C����Ԫ�أ�
D��Bͬ���ڣ���������D�ļ����Ӱ뾶��С����D����Ԫ�أ�
B��C��E��ɵ�36���ӵĻ�����Y�Ǽ�������������Ҫ�ɷ֣�����������Ҫ�ɷ��Ǵ������ƣ�����E����Ԫ�أ�
FԪ���������������۵Ĵ�����Ϊ4����F�Ƕ�����Ԫ�أ�����F����Ԫ�أ�
��1�����ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ����ǽ���Ԫ��֮�����γɹ��ۼ���
��2�����Ӳ���Խ������ӣ���뾶Խ���Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ���������������С��
��3������A��B��C��F����Ԫ�صĻ��������������ƺ����������ƣ��������Ƴ�ǿ�����ܺ����������Ʒ�Ӧ��
��4���������Ļ�����������ϡ�����������������������������������������������Һ�м����������������Һ����������������������ƫ��������Һ��������ϴ�ӡ�������պ�õ�һ�ֹ����������������������������������Ļ����������ȣ��������������൱����Ԫ�ص����������������������㼴�ɣ�
��5�����ݵ�ʧ�������д����Ӧ�Ļ�ѧ��Ӧ����ʽ�����ݷ�Ӧ����ʽ�����������Ƶ�������
��6���⻯�����Ҵ���Ӧ�����Ҵ��ƺ�������
����⣺��1�����������������Ӻʹ����������֮��������Ӽ������������������ԭ�Ӻ���ԭ��֮����ڹ��ۼ���
�ʴ�Ϊ�����Ӽ������ۼ������Թ��ۼ�����
��2�������ӵĵ��Ӳ���С�������ӡ������ӵĵ��Ӳ��������Ӳ���Խ�࣬���Ӱ뾶Խ�����������Ӱ뾶С�������Ӻ������Ӱ뾶�����Ӳ�����ͬ�����ӣ����Ӱ뾶����ԭ���������������С�����������Ӱ뾶���������Ӱ뾶��
�ʴ�Ϊ��S2-��Cl-��Al3+��
��3������������Һ��ǿ���ԣ�������������������ʽ�Σ������������ƺ����������Ʒ�Ӧ���������ơ�ˮ�Ͷ�������
�ʴ�Ϊ��H++HSO-3=SO2��+H2O��
��4���������Ļ�����������ϡ�����������������������������������������������Һ�м����������������Һ����������������������ƫ��������Һ��������ϴ�ӡ�������պ�õ�һ�ֹ����������������������������������Ļ����������ȣ��������������൱����Ԫ�ص�����������������������=
��100%=30%��
�ʴ�Ϊ��30%��
��5������ͼ��֪������������ӵ����ʵ���Ϊ0.2mol����������ӵ����ʵ���Ϊ0.1mol�����Դ���������ӵ����ʵ�������������ӵ����ʵ���֮��Ϊ2��1�����ݵ�ʧ�����غ�֪���������������Ƶķ�Ӧ����ʽΪ��10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O��
����Ҫ�������Ƶ�����Ϊx��
10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O
740g 2mol
x 0.1mol
x=37g
�ʴ�Ϊ��37g��10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O��
��6���⻯�����Ҵ���Ӧ�����Ҵ��ƺ��������ʴ�Ϊ��NaH+CH3CH2OH=CH3CH2ONa+H2����
�ʴ�Ϊ�����Ӽ������ۼ������Թ��ۼ�����
��2�������ӵĵ��Ӳ���С�������ӡ������ӵĵ��Ӳ��������Ӳ���Խ�࣬���Ӱ뾶Խ�����������Ӱ뾶С�������Ӻ������Ӱ뾶�����Ӳ�����ͬ�����ӣ����Ӱ뾶����ԭ���������������С�����������Ӱ뾶���������Ӱ뾶��
�ʴ�Ϊ��S2-��Cl-��Al3+��
��3������������Һ��ǿ���ԣ�������������������ʽ�Σ������������ƺ����������Ʒ�Ӧ���������ơ�ˮ�Ͷ�������
�ʴ�Ϊ��H++HSO-3=SO2��+H2O��
��4���������Ļ�����������ϡ�����������������������������������������������Һ�м����������������Һ����������������������ƫ��������Һ��������ϴ�ӡ�������պ�õ�һ�ֹ����������������������������������Ļ����������ȣ��������������൱����Ԫ�ص�����������������������=
| 16��3 |
| 16��3+56��2 |
�ʴ�Ϊ��30%��
��5������ͼ��֪������������ӵ����ʵ���Ϊ0.2mol����������ӵ����ʵ���Ϊ0.1mol�����Դ���������ӵ����ʵ�������������ӵ����ʵ���֮��Ϊ2��1�����ݵ�ʧ�����غ�֪���������������Ƶķ�Ӧ����ʽΪ��10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O��
����Ҫ�������Ƶ�����Ϊx��
10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O
740g 2mol
x 0.1mol
x=37g
�ʴ�Ϊ��37g��10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O��
��6���⻯�����Ҵ���Ӧ�����Ҵ��ƺ��������ʴ�Ϊ��NaH+CH3CH2OH=CH3CH2ONa+H2����
���������⿼����Ԫ�ص��ƶϡ����Ӱ뾶�İ뾶����ѧ�����жϵ�֪ʶ�㣬�״��ģ�4���⣬��ȷ�������е���Ԫ���൱����Ԫ���ǽⱾ��Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
��2011?����ģ�⣩�������ʵ�鲻�ܴﵽԤ��Ŀ���ǣ�������
|
