��Ŀ����
��8�֣�ʵ�����мס�����ƿ��ɫ��Һ������һƿ��ϡ���ᣬ��һƿ��̼������Һ��Ϊ�ⶨ�ס�����ƿ��Һ�ijɷּ����ʵ���Ũ�ȣ���������ʵ�飺
��ȡ25mL����Һ�������л�����������Һ15mL�����ռ���112mL����״�������塣
��ȡ15mL����Һ�������л����������Һ25mL�����ռ���56mL����״�������塣
��1���жϣ����� ��Һ������ ��Һ�������ѧʽ��
��2������Һ�����ʵ���Ũ��Ϊ mol/L������Һ�����ʵ���Ũ��
Ϊ mol/L�� ��Ҫ��д��������̣���
��ȡ25mL����Һ�������л�����������Һ15mL�����ռ���112mL����״�������塣
��ȡ15mL����Һ�������л����������Һ25mL�����ռ���56mL����״�������塣
��1���жϣ����� ��Һ������ ��Һ�������ѧʽ��
��2������Һ�����ʵ���Ũ��Ϊ mol/L������Һ�����ʵ���Ũ��
Ϊ mol/L�� ��Ҫ��д��������̣���
��1��HCl��1�֣� Na2CO3��1�֣�
��2�� 0.4��3�֣� 0.5��3�֣�
��2�� 0.4��3�֣� 0.5��3�֣�
��

��ϰ��ϵ�д�
 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
�����Ŀ
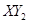 ����ס��ҵĻ�ѧʽ�ֱ������ �� ��
����ס��ҵĻ�ѧʽ�ֱ������ �� �� 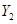 ��
��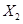
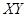 ��
��