��Ŀ����
��֪���� 2H2(g)��O2(g)===2H2O(g)����H����483.6 kJ��mol��1����H2(g)��S(g)=== H2S(g)����H����20.1 kJ��mol��1��
�����ж���ȷ����(����)
A��1 mol������ȫȼ������Һ̬ˮ��������241.8 kJ
B��1 mol H2O(g)��1 mol H2S(g)���������221.7 kJ
C���ɢ٢�֪��ˮ�����ȶ���С������
D������Ӧ���и��ù�̬��1 mol S(s)��ȫ��Ӧ���ų�������С��20.1 kJ
������ѡD�� A�1 mol������ȫȼ��������̬ˮ��������241.8 kJ��B�H2O(g)��1 mol H2S(g)���������Ƚϣ�C��ˮ�����ȶ��Ը������⡣

 С�����ϵ�д�
С�����ϵ�д���10L �����ܱ������г���X��g����Y(g)��������ӦX��g��+Y��g�� M��g��+N��g��������ʵ���������±���
M��g��+N��g��������ʵ���������±���
| ʵ�� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | |
| n(X) | n(Y) | n(M) | ||
| �� | 700 | 0.40 | 0.10 | 0.090 |
| �� | 800 | 0.10 | 0.40 | 0.080 |
| �� | 800 | 0.20 | 0.30 | a |
| �� | 900 | 0.10 | 0.15 | b |
����˵����ȷ���ǣ� ��
A��ʵ����У���5minʱ���n(M)=0.050mol����0��5minʱ���ڣ���N��ʾ��ƽ����Ӧ����v��N��=1.0��10-2mol/(L·min)
B��ʵ����У��÷�Ӧ��ƽ�ⳣ��K=2.0
C��ʵ����У��ﵽƽ��ʱ��X��ת����Ϊ60%
D��ʵ����У��ﵽƽ��ʱ��b>0.060
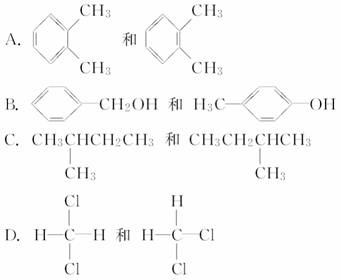
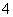 ��SO
��SO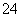 ��Cl��
��Cl��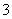 ��SO
��SO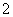 ����Һ�У�NH
����Һ�У�NH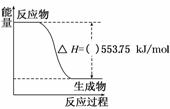
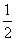 O2(g)===CO(g)�Ħ�H���������ʵ�顢���ø�˹���ɼ�����÷�Ӧ�Ħ�H������ʱ��Ҫ��õ�ʵ��������________��
O2(g)===CO(g)�Ħ�H���������ʵ�顢���ø�˹���ɼ�����÷�Ӧ�Ħ�H������ʱ��Ҫ��õ�ʵ��������________��