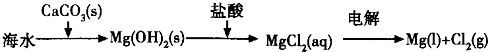
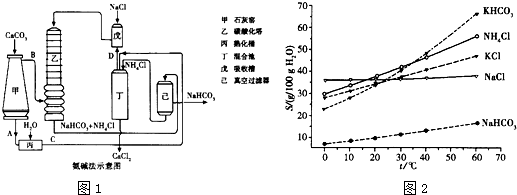
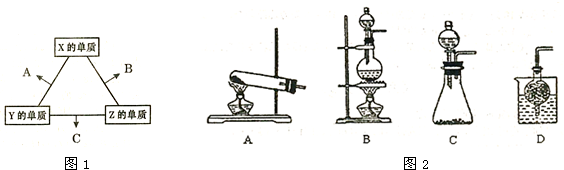
��Ŀ����
���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ���� ��
�����ᴿ���CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ�����ȣ�����CuCl�������Ʊ�CuCl�����ӷ���ʽ�� ��
28. (9��)����һ�仰������������ֵ��ǰ��ΪM������ΪN��M��N�Ĺ�ϵ��A��B��C��D��ѡ��
A. M>N B.M<N C.M=N D. ���Ƚ�
����ͬ�¶��£�1L 1mol/L ��NH4Cl��Һ�е�NH4��������2 L 0.5mol��L��1NH4Cl��Һ��NH4���ĸ����� ��
����ͬ�¶��£�pHֵΪ12���ռ���Һ��ˮ�ĵ���Ⱥ�pHֵΪ12��CH3COONa��Һ��ˮ�ĵ���ȣ� ��
����������ʱ�ı���ʯ��ˮ��һ�����µ�50�棻��һ�ݼ�������CaO���ָ������£�����Һ�е�c(Ca2+)�� ��
�ȳ��������ݵ�Ũ�ȵĴ�����Һ�����ڶ��������¶ȣ�����Һ��c(HCO3��)�� ��
�ɽ�pHֵΪ2�Ĵ�������ᶼϡ����ͬ��������ϡ��Һ��pHֵ�� ��
�ʳ�����0.1mol/L��CH3COOH��0.1mol/LCH3COONa�������Ϻ���Һ��c(Na+)��c(CH3COO��)�� ��
��ͬ�¶��£�0.1mol/LFeCl3��Һ��Fe3+ˮ��ٷ�����0.01mol��L��1FeCl3��Һ��Fe3+ ��ˮ��ٷ��ʣ� ��
��������ijǿ���ijǿ����Һ�������Ϻ���Һ��pHֵΪ7��ԭ����Һ��ԭ����Һ�����ʵ���Ũ�ȣ� ��
��PHֵ��ͬ�Ĵ�������ᣬ�ֱ�������ˮϡ����ԭ����M����N����ϡ�ͺ�����Һ��PHֵ��Ȼ��ͬ�� ��M��N�Ĺ�ϵ�ǣ� ��
����:��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�