��Ŀ����
(8�֣��л�����������ұ��ʷ������д���Ի͵�ƪ�£�������ǧ����ǰ������ʱ�ھ��С����������Ϊͭ���ļ��أ����ַ������ִ�ʪ��ұ�������������ͭ��Һ����������ʱ����������ͭ�����û���Ӧ�����ɽ���ͭ���÷�Ӧ�Ļ�ѧ����ʽΪFe+CuSO4=Cu+FeSO4��
���б���ԭ��Ԫ���� ����������Ԫ���� ���������� ����ԭ���� ������������ ����ԭ������ ��ÿ�õ�128��ͭʱ��ת�Ƶ�����ĿΪ ��
���б���ԭ��Ԫ���� ����������Ԫ���� ���������� ����ԭ���� ������������ ����ԭ������ ��ÿ�õ�128��ͭʱ��ת�Ƶ�����ĿΪ ��
Cu��Fe��CuSO4��Fe��FeSO4��Cu��2.408��1024
������������ϼ۽��ͱ���ԭ�����Ա���ԭ��Ԫ����Cu�����ϼ����߱����������Ա�������Ԫ����Fe���������۽��ͣ�����CuSO4�����������õ���Cu�ǻ�ԭ�����ԭ�������ߣ�����Fe�ǻ�ԭ�����õ���FeSO4���������1molCuת��6.02��1023���ӣ�����128��ͭת��2.408��1024�����ӡ�
�������������õ��Ӽ۽��ͱ���ԭ�õ���ԭ�����ԭ��ʧ���Ӽ����߱������õ��������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 CO��H2�����й��ڸ÷�Ӧ˵����ȷ����(����)
CO��H2�����й��ڸ÷�Ӧ˵����ȷ����(����) 
 ��1��ͼ�Т�Ϊ ����Ϊ ��
��1��ͼ�Т�Ϊ ����Ϊ ��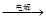 X+H2 ��Y+NaOH��G+W+H2O
X+H2 ��Y+NaOH��G+W+H2O  Cl2����MnCl2��2H2O
Cl2����MnCl2��2H2O