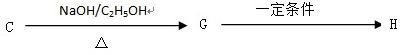��Ŀ����
��12�֣���֪��
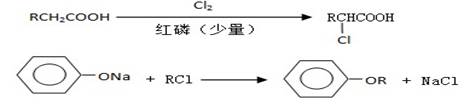
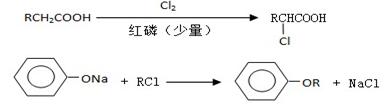
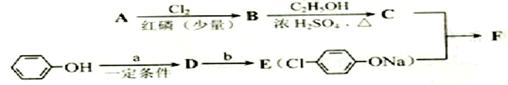
I.����ƽF�ǽ�Ѫ֬�������̴���ҩ�����һ���ϳ�·�����£�
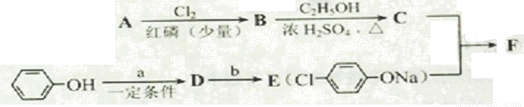
��1��AΪ����һԪ���ᣬ8.8g A������NaHCO3��Һ��Ӧ����2.24L CO2����״������A�ķ���ʽΪ_____________________________________________��
��2��д������A����ʽ�����м������Ľṹ��ʽ��
_________________________________________________________��
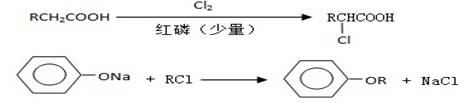
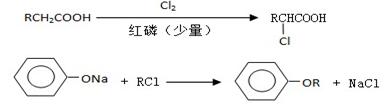
��3��B���ȴ����ᣬ��������ֻ��һ����ԭ�ӣ�д��B��C�ķ�Ӧ����ʽ��
__________________________________________________________��
��4��C+E��F�ķ�Ӧ����Ϊ________________________��
��5��д��A��F�Ľṹ��ʽ��
A______________________�� F__________________________��
���𰸡�
��1��C4H8O2����2�֣�
��2��HCOOCH2CH2CH3 HCOOCH(CH3)2����2�֣�д��һ����1�֣�ȫ�Ը�2�֣�
��3��(CH3)2CClCOOH+C2H5OH
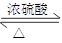 (CH3)2CClCOOC2H5+H2O����2�֣�
(CH3)2CClCOOC2H5+H2O����2�֣�
��4��ȡ����Ӧ��2�֣�
��5��(CH3)2CHCOOH
��2�֣�  ��2�֣�
��2�֣�
����������

��ϰ��ϵ�д�
�����Ŀ
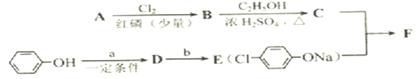
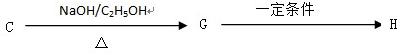
 H�ķ�Ӧ����ʽ��__________________________________
H�ķ�Ӧ����ʽ��__________________________________ ______________��
______________��
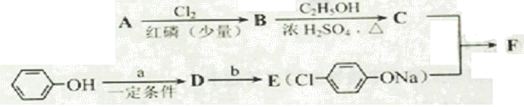
 2����״������A�ķ���ʽΪ________________��
2����״������A�ķ���ʽΪ________________�� __________________________________________��
__________________________________________��