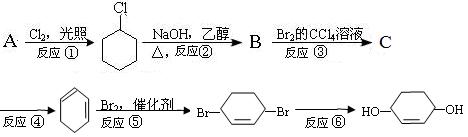
��Ŀ����
��10�֣�1��2-��������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ�2.18g��cm-3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�����ͼ4-3��ʾװ���Ʊ�1��2-�������顣���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Һ�壨���渲������ˮ������д���пհף�

(1)д���������Ʊ�1��2-���������������ѧ��Ӧ����ʽ______ ��
��2����ȫƿb���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱƿb�е�����___ ___��
��3������c��NaOH��Һ��������_____ _��
��4��ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³������ࡣ���װ�õ�������û�����⣬���ܵ�ԭ���Т���ϩ����(��ͨ��Һ��)���ʹ��죬�� ��

(1)д���������Ʊ�1��2-���������������ѧ��Ӧ����ʽ______ ��
��2����ȫƿb���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱƿb�е�����___ ___��
��3������c��NaOH��Һ��������_____ _��
��4��ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³������ࡣ���װ�õ�������û�����⣬���ܵ�ԭ���Т���ϩ����(��ͨ��Һ��)���ʹ��죬�� ��
��10�֣���1��CH3CH2OH 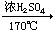 CH2 =CH2��+H2O CH2=CH2+Br2��CH2BrCH2Br
CH2 =CH2��+H2O CH2=CH2+Br2��CH2BrCH2Br
��2��b��ˮ����½����������е�ˮ�������������������
��3����ȥ��ϩ�д��е������������ȥCO2��SO2��
��4����ʵ������У��Ҵ���Ũ����Ļ��Һû��Ѹ�ٴﵽ170�棨�𡰿��²�������ɣ���
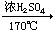 CH2 =CH2��+H2O CH2=CH2+Br2��CH2BrCH2Br
CH2 =CH2��+H2O CH2=CH2+Br2��CH2BrCH2Br��2��b��ˮ����½����������е�ˮ�������������������
��3����ȥ��ϩ�д��е������������ȥCO2��SO2��
��4����ʵ������У��Ҵ���Ũ����Ļ��Һû��Ѹ�ٴﵽ170�棨�𡰿��²�������ɣ���
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
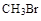
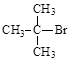
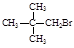
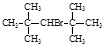
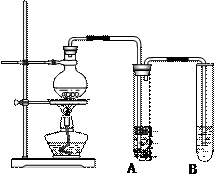
 ��1:1�����ᡣ���������1:1���������õ�����Ϊ
��1:1�����ᡣ���������1:1���������õ�����Ϊ �����ﵼ��ʢ�б�ˮ�������Թ�A�У���ˮ������������ ��
�����ﵼ��ʢ�б�ˮ�������Թ�A�У���ˮ������������ �� ��ѡ���ţ���
��ѡ���ţ��� ��������Һϴ�� c�������Ȼ�̼��ȡ d��������������Һϴ��
��������Һϴ�� c�������Ȼ�̼��ȡ d��������������Һϴ��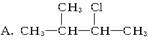 B��CH3CH2Br
B��CH3CH2Br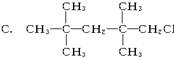 D��CH2ClCH2CH3
D��CH2ClCH2CH3