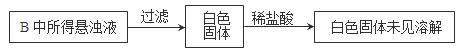
��Ŀ����
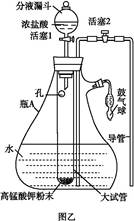
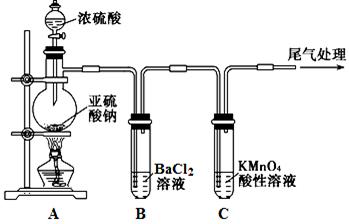
ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣
��ش��������⣺
��1��װ��A��ʢ���������Ƶ�����������__________________�����з�����Ӧ�Ļ�ѧ����ʽΪ______________��
��2��ʵ������У�װ��B��C�в���������ֱ���___________��___________����Щ����ֱ�˵��SO2���е�������___________��___________��װ��B�з�����Ӧ�����ӷ���ʽΪ_________________________________________________��
��3��װ��D��Ŀ����̽��SO2��Ʒ����Һ���õĿ����ԣ���д��ʵ�����������____________��
��4��β���ɲ���______________��Һ���ա�
��1��������ƿ
Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O
��2����Һ���Ϻ�ɫ��Ϊ��ɫ ��ɫ��Һ�г��ֻ�ɫ���� ��ԭ�� ������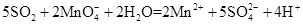
��3��Ʒ����Һ��ɫ�رշ�Һ©������������ȼ�ƾ��Ƽ��ȣ�Ʒ����Һ�ָ�Ϊ��ɫ
��4��NaOH���𰸺������ɣ�
����

����(H2S)��һ�־��г�������ζ����ɫ���壬�о綾�������ڶ������������Լ���Ȼ���С�������ĺܶ�����������Ҳ������Ҫ���á�
���ϣ���H2S������ˮ?Լ1��2?����ˮ��ҺΪ��Ԫ���ᡣ
��H2S��������������ӷ�Ӧ���ɳ�����
��H2S�ڿ�����ȼ�գ�����ʵ���ɫ��
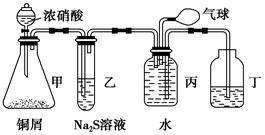
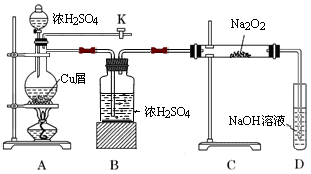
(1)ij��ѧС���������ȡH2S����֤�����ʵ�ʵ�飬����ͼ��ʾ��A����CuSO4��Һ��B�з���ʪ�����ɫʯ����ֽ��C����FeCl3��Һ��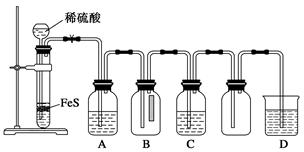
�ش��������⣺
��A���к�ɫ����(CuS)������A�з�����Ӧ�Ļ�ѧ����ʽΪ___________________��
��B�������__________________��
��C��ֻ��dz��ɫ��������������Һ��dz��ɫ����C�з�����Ӧ�����ӷ���ʽΪ______________��
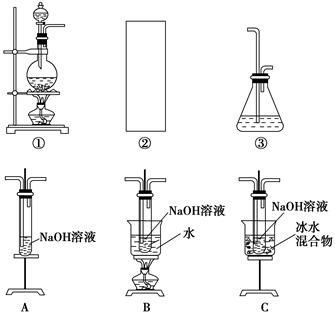
��D��ʢ�ŵ��Լ�������________(����ĸ���)��
a��ˮ b������
c��NaCl��Һ d��NaOH��Һ
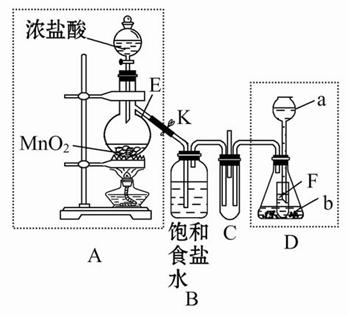
(2)Ϊ��һ��̽����2����Ļ������룫4����Ļ����ﷴӦ������С��ͬѧ�����������ʵ�顣
| | ʵ����� | ʵ������ |
| ʵ��1 | ����Ũ�ȵ�Na2S��Na2SO3��Һ�������2��1��� | ���������� |
| ʵ��2 | ��H2Sͨ��Na2SO3��Һ�� | δ�����Գ������ټ�������ϡ���ᣬ������������dz��ɫ���� |
| ʵ��3 | ��SO2ͨ��Na2S��Һ�� | ��dz��ɫ�������� |
��֪������ƽ�ⳣ����
H2S��Kal��1.3��10��7��Ka2��7.1��10��15
H2SO3��Ka1��1.7��10��2��Ka2��5.6��10��8
�ٸ�������ʵ�飬���Եó����ۣ���__________�����£���4���������������2����Ļ����
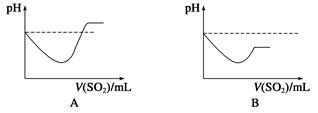
�ڽ�SO2����ͨ��H2Sˮ��Һ��ֱ�����������б�ʾ��ҺpH��SO2��������仯��ϵʾ��ͼ��ȷ����________(����ĸ���)��

(3)�����أ�������H2S����Ag�����û���Ӧ����H2���ֽ�H2S����ͨ��װ�����۵IJ����ܣ�����Ƽ�ʵ�飬ͨ�����鷴Ӧ����֤��H2S��Ag�������û���Ӧ______��
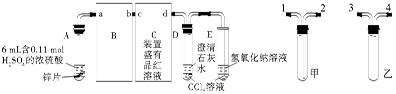
 Na2SO4��SO2����H2O
Na2SO4��SO2����H2O