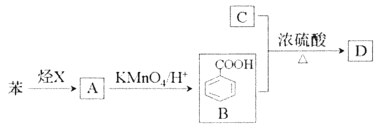
��Ŀ����
����Ŀ����֪�١����Ԫ�������ڱ��е�λ����ͼ���Իش��������⣺

(1)����Ԫ��������d�����У�________(����)��
(2)�ڡ��ۡ�������Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ________(��Ԫ�ط��ű�ʾ)��
(3)����ݵȵ�����ԭ����д���ɢڡ�������Ԫ����ɵĴ���һ����λ��������ӵĵ���ʽ��_____��
(4)���Ԫ����Ԫ�����ڱ��е�λ����___�����ԭ�Ӵ��ڻ�̬ʱ��������Ų�ʽΪ____����֪Ԫ�آ�͢ߵĵ縺�Էֱ�Ϊ1.9��2.5���������γɵĻ���������________(����ӡ����ۡ�)�����
(5)�ۺ�Ԫ��ԭ����ٺ�Ԫ��ԭ���γɵ�ԭ�Ӹ�����Ϊ1��3�ķ���X�Ŀռ乹��Ϊ________��X�ڢ�����γɵĻ�����Y�е��ܽ�Ⱥܴ�����Ҫԭ����___________��X����������ԭ��Ϊ________�ӻ���X��������Ԫ�ض�Ӧ�Ķ����������γɵ������ӵĻ�ѧʽΪ______________��
(6)�ݺ�Ԫ��ԭ����TlԪ���γɵľ���ľ�����ͼ��ʾ�������ʵĻ�ѧʽΪ______________��������Tlԭ�ӣ���˾����ТݵĿռ�ṹ�����ֳ������ʵľ���ṹһ����______________����֪�þ�����ܶ�Ϊ�� g��cm��3�������ӵ�����ΪNA����þ����о����߳�Ϊ________ pm(ֻд����ʽ)��

���𰸡���� )N>O>C [��CN��]�� ��4���ڵڢ��� 1s22s22p63s23p63d104s1 ���� ������ ˮ�Ͱ������Ӽ����γ������ˮ�Ͱ������Ӷ��Ǽ��Է��� sp3 [Cu(NH3)4]2�� NaTl ���ʯ ![]()
��������
��1��d��������B����B���Լ�����Ԫ�أ����������������ڱ���λ��d����Ԫ���Ǣ�
��2����ΪC����ΪN����ΪO��ͬ���ڴ������ҵ�һ��������������A>��A����A>��A�����˳����N>O>C��
��3��������CN����C��N֮�乲���������Ӷԣ������ʽΪ![]() ��
��
��4�����Ԫ��ΪFe��λ�ڵ������ڢ��壻���Ԫ��ΪCu����̬��������Ų�ʽΪ1s22s22p63s23p63d104s1���ߺ�Ԫ��ΪS������Ԫ�ص縺�Բ�ֵΪ(2.5��1.9)=0.6<1.7����ԭ�Ӽ乹�ɹ��ۼ������û�����Ϊ���ۻ����
��5���ۺ�Ԫ��ΪN���ٺ�Ԫ��ΪH��ԭ�Ӹ�����Ϊ1��3�����û�����ΪNH3���ռ乹��Ϊ�����Σ��٢��γɵĻ�����ΪH2O��NH3��ˮ���ܽ�ȴ��ԭ����NH3��H2O���Ǽ��Է��ӣ�NH3��H2O�γɷ��Ӽ����������NH3���ܽ�ȣ�NH3������ԭ��N��3���Ҽ����µ��Ӷ���Ϊ(5��3��1)/2=1���۲���Ӷ���Ϊ4���ӻ�����Ϊsp3��NH3��Cu2���γ������ӣ�Cu2���������ӣ���λ��Ϊ4���仯ѧʽΪ[Cu(NH3)4]2����
��6���ݺ�Ԫ��ΪNa�����ݾ����ṹ��Naλ�ھ��������ϡ������ڲ�������Ϊ12��1/4��5=8��Tiλ�ھ����Ķ��㡢���ĺ��ڲ�������Ϊ8��18��6��1/2��4=8����ѧʽΪNaTi������Tiԭ�ӣ��ռ乹������ʯ�Ľṹһ��������������Ϊ![]() �����������Ϊ(a��10��10)cm3�������ܶȵĶ��徧���ı߳�Ϊ
�����������Ϊ(a��10��10)cm3�������ܶȵĶ��徧���ı߳�Ϊ![]() pm��
pm��

����Ŀ��ijУ��ѧ��ȤС��Ϊ�о����������ʲ�ģ�ҵ�Ʊ�Ư�ۣ����������װ�ý���ʵ�顣��֪����A�з�ӦΪ KClO3��6HCl(Ũ)=KCl��3Cl2����3H2O��
��ʯ�������Ҫ�ɷ�ΪCa(OH)2���������ʲ����뷴Ӧ��

��1��Bװ������____��ʵ������������� B ����Һ�μ�������ɫʯ����ֽ�ϣ��ɹ۲쵽��������_____��
��2��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III�����η����������ȷ����___�����ţ���
��� | I | II | III |
A | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
B | �������ɫ���� | Ũ���� | ʪ�����ɫ���� |
C | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
D | ʪ�����ɫ���� | ��ʯ�� | �������ɫ���� |
��3����E��������ȫ��Ӧ����һϵ�мӹ��������õ�Ư����Ʒ������Ҫ�ɷ�Ϊ___���ѧʽ��
��4��Fװ�õ������ǣ��û�ѧ����ʽ��ʾ��____��
��5��Ϊ�ⶨ��3��������Ư�۵���Ч�ɷݺ�������ȡagƯ����Ʒ�ܽ⣬��������Һ��ͨ�� CO2�������������ֵʱ���ù��̵Ļ�ѧ����ʽΪ_____������Ӧ���ɳ��������ʵ���Ϊbmol�����Ư������Ч�ɷݵ���������Ϊ_____���ú�a��b��ʽ�ӱ�ʾ����